A disulfide bond in the TIM23 complex is crucial for voltage gating and mitochondrial protein import
- PMID: 27502485
- PMCID: PMC4987294
- DOI: 10.1083/jcb.201602074
A disulfide bond in the TIM23 complex is crucial for voltage gating and mitochondrial protein import
Abstract
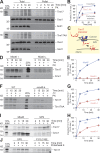
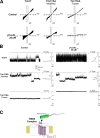
Tim17 is a central, membrane-embedded subunit of the mitochondrial protein import machinery. In this study, we show that Tim17 contains a pair of highly conserved cysteine residues that form a structural disulfide bond exposed to the intermembrane space (IMS). This disulfide bond is critical for efficient protein translocation through the TIM23 complex and for dynamic gating of its preprotein-conducting channel. The disulfide bond in Tim17 is formed during insertion of the protein into the inner membrane. Whereas the import of Tim17 depends on the binding to the IMS protein Mia40, the oxidoreductase activity of Mia40 is surprisingly dispensable for Tim17 oxidation. Our observations suggest that Tim17 can be directly oxidized by the sulfhydryl oxidase Erv1. Thus, import and oxidation of Tim17 are mediated by the mitochondrial disulfide relay, though the mechanism by which the disulfide bond in Tim17 is formed differs considerably from that of soluble IMS proteins.
© 2016 Ramesh et al.
Figures





References
-
- Bode M., Woellhaf M.W., Bohnert M., van der Laan M., Sommer F., Jung M., Zimmermann R., Schroda M., and Herrmann J.M.. 2015. Redox-regulated dynamic interplay between Cox19 and the copper-binding protein Cox11 in the intermembrane space of mitochondria facilitates biogenesis of cytochrome c oxidase. Mol. Biol. Cell. 26:2385–2401. 10.1091/mbc.E14-11-1526 - DOI - PMC - PubMed
-
- Chacinska A., Pfannschmidt S., Wiedemann N., Kozjak V., Sanjuán Szklarz L.K., Schulze-Specking A., Truscott K.N., Guiard B., Meisinger C., and Pfanner N.. 2004. Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J. 23:3735–3746. 10.1038/sj.emboj.7600389 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

