Multi-site phospho-regulation of proneural transcription factors controls proliferation versus differentiation in development and reprogramming
- PMID: 27502783
- PMCID: PMC4973605
- DOI: 10.1080/23262133.2015.1049733
Multi-site phospho-regulation of proneural transcription factors controls proliferation versus differentiation in development and reprogramming
Abstract
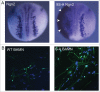
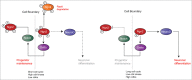
During development of the nervous system, it is essential to co-ordinate the processes of proliferation and differentiation. Basic helix-loop-helix transcription factors play a central role in controlling neuronal differentiation and maturation as well as being components of the combinatorial code that determines neuronal identity. We have recently shown that the ability of the proneural proteins Ngn2 and Ascl1 to drive neuronal differentiation is inhibited by cyclin dependent kinase-mediated multi-site phosphorylation. This limits downstream target promoter dwell time, thus demonstrating a direct mechanistic regulatory link between the cell cycle and differentiation machinery.Proneural proteins are key components of transcription factor cocktails that can bring about the direct reprogramming of human fibroblasts into neurons. Building on our observations demonstrating that phospho-mutant proneural proteins show an enhanced ability to drive neuronal differentiation in vivo, we see that replacing wild-type with phospho-mutant proneural proteins in fibroblast reprogramming cocktails significantly enhances the axonal outgrowth, branching and electrophysiological maturity of the neurons generated. A model is presented here that can explain the enhanced ability of dephosphorylated proneural proteins to drive neuronal differentiation, and some unanswered questions in this emerging area are highlighted.
Keywords: Xenopus; cell cycle; cyclin-dependent kinase; development; differentiation; phosphorylation; proneural; reprogramming; transcription factor.
Figures
Comment on
-
The phosphorylation status of Ascl1 is a key determinant of neuronal differentiation and maturation in vivo and in vitro.Development. 2014 Jun;141(11):2216-24. doi: 10.1242/dev.106377. Epub 2014 May 12. Development. 2014. PMID: 24821983
References
-
- Salomoni P, Calegari F. Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol 2010; 20:233-43; PMID:20153966; http://dx.doi.org/10.1016/j.tcb.2010.01.006 - DOI - PubMed
-
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci 2002; 3:517-30; PMID:12094208; http://dx.doi.org/10.1038/nrn874 - DOI - PubMed
-
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010; 463:1035-41; PMID:20107439; http://dx.doi.org/10.1038/nature08797 - DOI - PMC - PubMed
-
- Yang N, Ng YH, Pang ZP, Sudhof TC, Wernig M. Induced neuronal cells: how to make and define a neuron. Cell Stem Cell 2011; 9:517-25; PMID:22136927; http://dx.doi.org/10.1016/j.stem.2011.11.015 - DOI - PMC - PubMed
-
- Ali F, Hindley C, McDowell G, Deibler R, Jones A, Kirschner M, Guillemot F, Philpott A. Cell cycle-regulated multi-site phosphorylation of Neurogenin 2 coordinates cell cycling with differentiation during neurogenesis. Development 2011; 138:4267-77; PMID:21852393; http://dx.doi.org/10.1242/dev.067900 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
