Classical Galactosaemia and CDG, the N-Glycosylation Interface. A Review
- PMID: 27502837
- PMCID: PMC5509556
- DOI: 10.1007/8904_2016_5
Classical Galactosaemia and CDG, the N-Glycosylation Interface. A Review
Abstract
Classical galactosaemia is a rare disorder of carbohydrate metabolism caused by galactose-1-phosphate uridyltransferase (GALT) deficiency (EC 2.7.7.12). The disease is life threatening if left untreated in neonates and the only available treatment option is a long-term galactose restricted diet. While this is lifesaving in the neonate, complications persist in treated individuals, and the cause of these, despite early initiation of treatment, and shared GALT genotypes remain poorly understood. Systemic abnormal glycosylation has been proposed to contribute substantially to the ongoing pathophysiology. The gross N-glycosylation assembly defects observed in the untreated neonate correct over time with treatment. However, N-glycosylation processing defects persist in treated children and adults.Congenital disorders of glycosylation (CDG) are a large group of over 100 inherited disorders affecting largely N- and O-glycosylation.In this review, we compare the clinical features observed in galactosaemia with a number of predominant CDG conditions.We also summarize the N-glycosylation abnormalities, which we have described in galactosaemia adult and paediatric patients, using an automated high-throughput HILIC-UPLC analysis of galactose incorporation into serum IgG with analysis of the corresponding N-glycan gene expression patterns and the affected pathways.
Figures
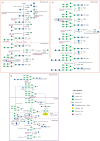
References
-
- Barone R, Pavone V, Pennisi P, Fiumara A, Fiore CE. Assessment of skeletal status in patients with congenital disorder of glycosylation type IA. Int J Tissue React. 2002;24:23–28. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

