An activated form of ADAM10 is tumor selective and regulates cancer stem-like cells and tumor growth
- PMID: 27503072
- PMCID: PMC4995075
- DOI: 10.1084/jem.20151095
An activated form of ADAM10 is tumor selective and regulates cancer stem-like cells and tumor growth
Abstract
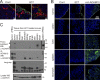
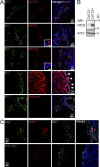
The transmembrane metalloprotease ADAM10 sheds a range of cell surface proteins, including ligands and receptors of the Notch, Eph, and erbB families, thereby activating signaling pathways critical for tumor initiation and maintenance. ADAM10 is thus a promising therapeutic target. Although widely expressed, its activity is normally tightly regulated. We now report prevalence of an active form of ADAM10 in tumors compared with normal tissues, in mouse models and humans, identified by our conformation-specific antibody mAb 8C7. Structure/function experiments indicate mAb 8C7 binds an active conformation dependent on disulfide isomerization and oxidative conditions, common in tumors. Moreover, this active ADAM10 form marks cancer stem-like cells with active Notch signaling, known to mediate chemoresistance. Importantly, specific targeting of active ADAM10 with 8C7 inhibits Notch activity and tumor growth in mouse models, particularly regrowth after chemotherapy. Our results indicate targeted inhibition of active ADAM10 as a potential therapy for ADAM10-dependent tumor development and drug resistance.
© 2016 Atapattu et al.
Figures








References
-
- Anders A., Gilbert S., Garten W., Postina R., and Fahrenholz F.. 2001. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 15:1837–1839. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

