Novel antibodies to phosphorylated α-synuclein serine 129 and NFL serine 473 demonstrate the close molecular homology of these epitopes
- PMID: 27503460
- PMCID: PMC4977832
- DOI: 10.1186/s40478-016-0357-9
Novel antibodies to phosphorylated α-synuclein serine 129 and NFL serine 473 demonstrate the close molecular homology of these epitopes
Abstract
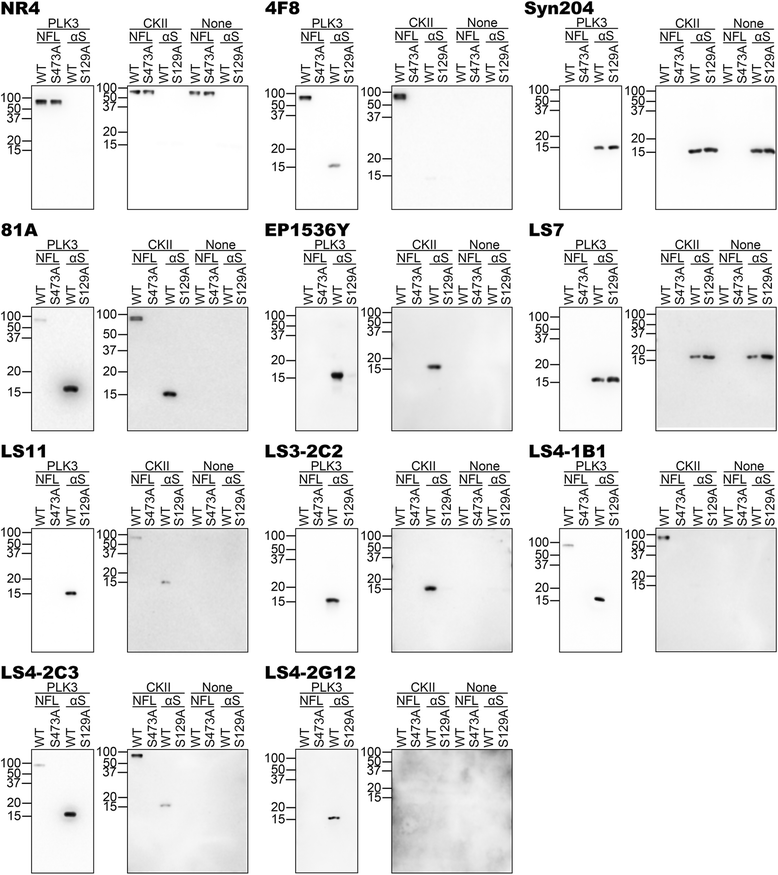
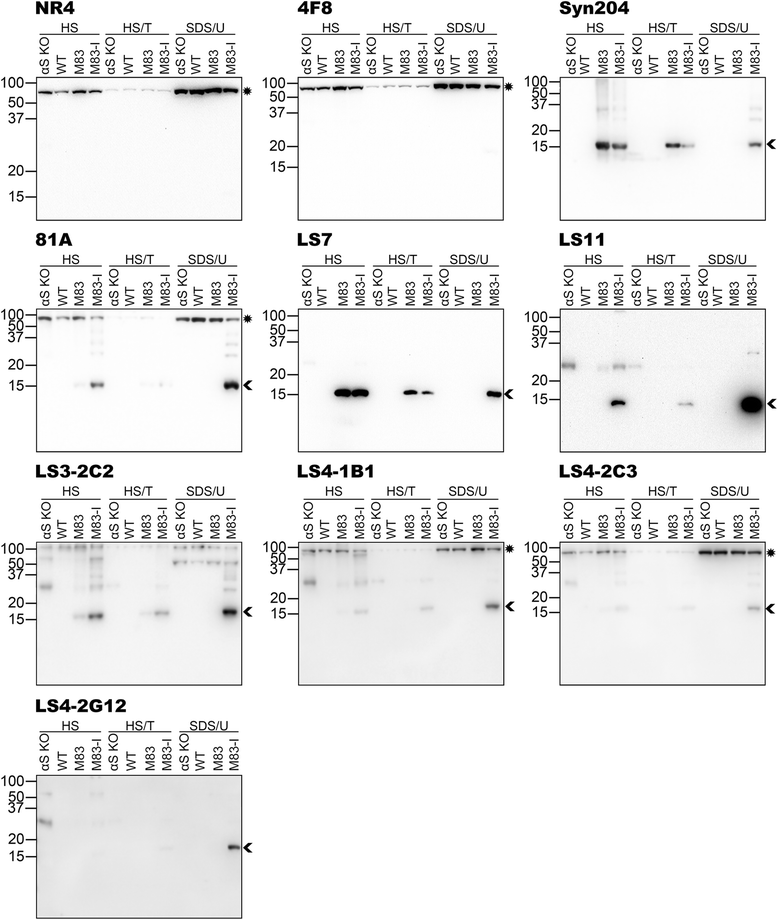
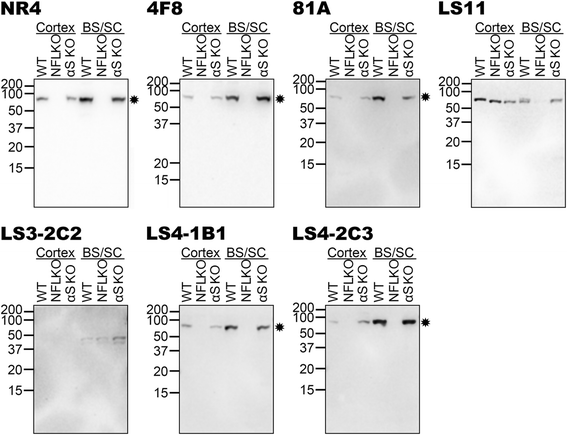
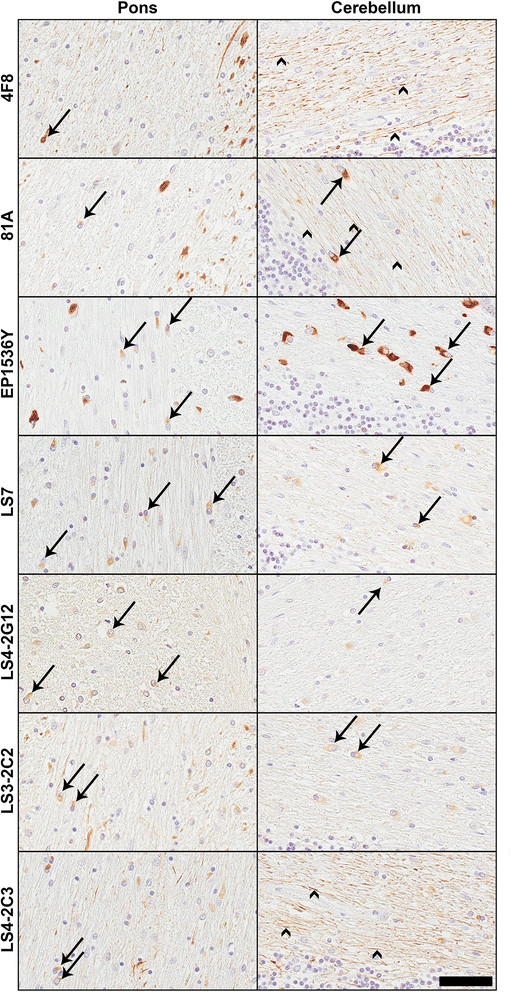
Pathological inclusions containing aggregated, highly phosphorylated (at serine129) α-synuclein (αS pSer129) are characteristic of a group of neurodegenerative diseases termed synucleinopathies. Antibodies to the pSer129 epitope can be highly sensitive in detecting αS inclusions in human tissue and experimental models of synucleinopathies. However, the generation of extensively specific pSer129 antibodies has been problematic, in some cases leading to the misinterpretation of αS inclusion pathology. One common issue is cross-reactivity to the low molecular mass neurofilament subunit (NFL) phosphorylated at Ser473. Here, we generated a series of monoclonal antibodies to the pSer129 αS and pSer473 NFL epitopes. We determined the relative abilities of the known αS kinases, polo-like kinases (PLK) 1, 2 and 3 and casein kinase (CK) II in phosphorylating NFL and αS, while using this information to characterize the specificity of the new antibodies. NFL can be phosphorylated by PLK1, 2 and 3 at Ser473; however CKII shows the highest phosphorylation efficiency and specificity for this site. Conversely, PLK3 is the most efficient kinase at phosphorylating αS at Ser129, but there is overlay in the ability of these kinases to phosphorylate both epitopes. Antibody 4F8, generated to the pSer473 NFL epitope, was relatively specific for phosphorylated NFL, however it could uniquely cross-react with pSer129 αS when highly phosphorylated, further showing the structural similarity between these phospho-epitopes. All of the new pSer129 antibodies detected pathological αS inclusions in human brains and mouse and cultured cell experimental models of induced synucleinopathies. Several of these pSer129 αS antibodies reacted with the pSer473 NFL epitope, but 2 clones (LS3-2C2 and LS4-2G12) did not. However, LS3-2C2 demonstrated cross-reactivity with other proteins. Our findings further demonstrate the difficulties in generating specific pSer129 αS antibodies, but highlights that the use of multiple antibodies, such as those generated here, can provide a sensitive and accurate assessment of αS pathology.
Keywords: Monoclonal antibodies; Neurofilament; Parkinson’s disease; Phosphorylation; α-synuclein.
Figures






Similar articles
-
In vivo modulation of polo-like kinases supports a key role for PLK2 in Ser129 α-synuclein phosphorylation in mouse brain.Neuroscience. 2014 Jan 3;256:72-82. doi: 10.1016/j.neuroscience.2013.09.061. Epub 2013 Oct 12. Neuroscience. 2014. PMID: 24128992
-
Nuclear and neuritic distribution of serine-129 phosphorylated alpha-synuclein in transgenic mice.Neuroscience. 2009 Jun 2;160(4):796-804. doi: 10.1016/j.neuroscience.2009.03.002. Epub 2009 Mar 9. Neuroscience. 2009. PMID: 19272424
-
Amyloidogenic α-synuclein seeds do not invariably induce rapid, widespread pathology in mice.Acta Neuropathol. 2014 May;127(5):645-65. doi: 10.1007/s00401-014-1268-0. Acta Neuropathol. 2014. PMID: 24659240 Free PMC article.
-
Antibodies against alpha-synuclein: tools and therapies.J Neurochem. 2019 Sep;150(5):612-625. doi: 10.1111/jnc.14713. Epub 2019 Jun 25. J Neurochem. 2019. PMID: 31055836 Review.
-
α-Synuclein phosphorylation as a therapeutic target in Parkinson's disease.Rev Neurosci. 2012 Mar 21;23(2):191-8. doi: 10.1515/revneuro-2011-0067. Rev Neurosci. 2012. PMID: 22499677 Review.
Cited by
-
Revisiting the specificity and ability of phospho-S129 antibodies to capture alpha-synuclein biochemical and pathological diversity.NPJ Parkinsons Dis. 2022 Oct 20;8(1):136. doi: 10.1038/s41531-022-00388-7. NPJ Parkinsons Dis. 2022. PMID: 36266318 Free PMC article.
-
Novel Conformation-Dependent Tau Antibodies Are Modulated by Adjacent Phosphorylation Sites.Int J Mol Sci. 2023 Sep 5;24(18):13676. doi: 10.3390/ijms241813676. Int J Mol Sci. 2023. PMID: 37761979 Free PMC article.
-
Defining α-synuclein species responsible for Parkinson's disease phenotypes in mice.J Biol Chem. 2019 Jul 5;294(27):10392-10406. doi: 10.1074/jbc.RA119.007743. Epub 2019 May 29. J Biol Chem. 2019. PMID: 31142553 Free PMC article.
-
Propagation of α-Synuclein Strains within Human Reconstructed Neuronal Network.Stem Cell Reports. 2019 Feb 12;12(2):230-244. doi: 10.1016/j.stemcr.2018.12.007. Epub 2019 Jan 10. Stem Cell Reports. 2019. PMID: 30639210 Free PMC article.
-
α-Synuclein fibril-induced paradoxical structural and functional defects in hippocampal neurons.Acta Neuropathol Commun. 2018 May 1;6(1):35. doi: 10.1186/s40478-018-0537-x. Acta Neuropathol Commun. 2018. PMID: 29716652 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

