Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib
- PMID: 27503501
- PMCID: PMC5064717
- DOI: 10.1182/blood-2016-06-722991
Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib
Abstract
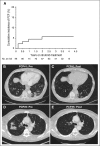
Ibrutinib is not known to confer risk for Pneumocystis jirovecii pneumonia (PCP). We observed 5 cases of PCP in 96 patients receiving single-agent ibrutinib, including 4 previously untreated. Clinical presentations included asymptomatic pulmonary infiltrates, chronic cough, and shortness of breath. The diagnosis was often delayed. Median time from starting ibrutinib to occurrence of PCP was 6 months (range, 2-24). The estimated incidence of PCP was 2.05 cases per 100 patient-years (95% confidence interval, 0.67-4.79). At the time of PCP, all patients had CD4 T-cell count >500/μL (median, 966/μL) and immunoglobulin G (IgG) >500 mg/dL (median, 727 mg/dL). All patients underwent bronchoalveolar lavage. P jirovecii was identified by polymerase chain reaction in all 5 cases; direct fluorescence antibody staining was positive in 1. All events were grade ≤2 and resolved with oral therapy. Secondary prophylaxis was not given to 3 patients; after 61 patient-months of follow up, no recurrence occurred. Lack of correlation with CD4 count and IgG level suggests that susceptibility to PCP may be linked to Bruton tyrosine kinase (BTK) inhibition. If confirmed, this association could result in significant changes in surveillance and/or prophylaxis, possibly extending to other BTK inhibitors. This trial was registered at www.clinicaltrials.gov as #NCT01500733 and #NCT02514083.
Figures
Comment in
-
CLL: an acquired immunodeficiency disease.Blood. 2016 Oct 13;128(15):1908-1909. doi: 10.1182/blood-2016-08-734475. Blood. 2016. PMID: 27737845 No abstract available.
References
-
- Eichhorst B, Fink AM, Bahlo J, et al. International Group of Investigators; German CLL Study Group (GCLLSG) First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–942. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. International Group of Investigators; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

