Tristetraprolin as a Therapeutic Target in Inflammatory Disease
- PMID: 27503556
- PMCID: PMC5030171
- DOI: 10.1016/j.tips.2016.07.002
Tristetraprolin as a Therapeutic Target in Inflammatory Disease
Abstract
Members of the tristetraprolin (TTP) family of RNA-binding proteins are found in all major eukaryotic groups. TTP family members, from plants through humans, can bind adenosine-uridine rich elements in target mRNAs with high affinity. In mammalian cells, these proteins then promote deadenylation and decay of target transcripts. Four such proteins are found in mice, of which the best studied is TTP. When the gene encoding TTP is disrupted in mice, the animals develop a severe syndrome of arthritis, autoimmunity, cachexia, dermatitis, and myeloid hyperplasia. Conversely, recent overexpression studies have demonstrated protection against several experimental models of immune inflammatory disease. This endogenous anti-inflammatory protein could serve as the basis for novel approaches to therapy of similar conditions in humans.
Keywords: autoimmunity; deadenylation; inflammation; mRNA decay; tumor necrosis factor.
Published by Elsevier Ltd.
Figures



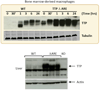
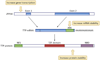
References
-
- Palladino MA, et al. Anti-TNF-alpha therapies: the next generation. Nature reviews. Drug discovery. 2003;2:736–746. - PubMed
-
- Ramiro S, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Annals of the rheumatic diseases. 2014;73:529–535. - PubMed
-
- Carballo E, et al. Bone marrow transplantation reproduces the tristetraprolin-deficiency syndrome in recombination activating gene-2 (−/−) mice. Evidence that monocyte/macrophage progenitors may be responsible for TNFalpha overproduction. The Journal of clinical investigation. 1997;100:986–995. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

