Vacuolar Chloride Fluxes Impact Ion Content and Distribution during Early Salinity Stress
- PMID: 27503602
- PMCID: PMC5047071
- DOI: 10.1104/pp.16.00183
Vacuolar Chloride Fluxes Impact Ion Content and Distribution during Early Salinity Stress
Abstract
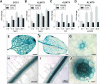
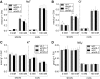
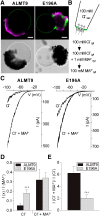
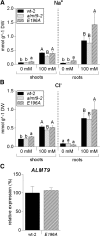
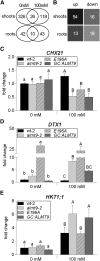
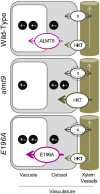
The ability to control the cytoplasmic environment is a prerequisite for plants to cope with changing environmental conditions. During salt stress, for instance, Na+ and Cl- are sequestered into the vacuole to help maintain cytosolic ion homeostasis and avoid cellular damage. It has been observed that vacuolar ion uptake is tied to fluxes across the plasma membrane. The coordination of both transport processes and relative contribution to plant adaptation, however, is still poorly understood. To investigate the link between vacuolar anion uptake and whole-plant ion distribution during salinity, we used mutants of the only vacuolar Cl- channel described to date: the Arabidopsis (Arabidopsis thaliana) ALMT9. After 24-h NaCl treatment, almt9 knock-out mutants had reduced shoot accumulation of both Cl- and Na+ In contrast, almt9 plants complemented with a mutant variant of ALMT9 that exhibits enhanced channel activity showed higher Cl- and Na+ accumulation. The altered shoot ion contents were not based on differences in transpiration, pointing to a vacuolar function in regulating xylem loading during salinity. In line with this finding, GUS staining demonstrated that ALMT9 is highly expressed in the vasculature of shoots and roots. RNA-seq analysis of almt9 mutants under salinity revealed specific expression profiles of transporters involved in long-distance ion translocation. Taken together, our study uncovers that the capacity of vacuolar Cl- loading in vascular cells plays a crucial role in controlling whole-plant ion movement rapidly after onset of salinity.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures
References
-
- Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics International 11: 36–42
-
- Arrivault S, Senger T, Krämer U (2006) The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J 46: 861–879 - PubMed
-
- Barbier-Brygoo H, De Angeli A, Filleur S, Frachisse JM, Gambale F, Thomine S, Wege S (2011) Anion channels/transporters in plants: from molecular bases to regulatory networks. Annu Rev Plant Biol 62: 25–51 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

