Chronic Iron Limitation Confers Transient Resistance to Oxidative Stress in Marine Diatoms
- PMID: 27503604
- PMCID: PMC5047098
- DOI: 10.1104/pp.16.00840
Chronic Iron Limitation Confers Transient Resistance to Oxidative Stress in Marine Diatoms
Abstract
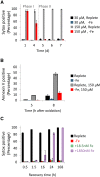
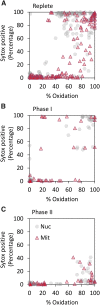
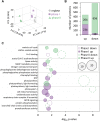
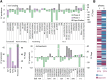
Diatoms are single-celled, photosynthetic, bloom-forming algae that are responsible for at least 20% of global primary production. Nevertheless, more than 30% of the oceans are considered "ocean deserts" due to iron limitation. We used the diatom Phaeodactylum tricornutum as a model system to explore diatom's response to iron limitation and its interplay with susceptibility to oxidative stress. By analyzing physiological parameters and proteome profiling, we defined two distinct phases: short-term (<3 d, phase I) and chronic (>5 d, phase II) iron limitation. While at phase I no significant changes in physiological parameters were observed, molecular markers for iron starvation, such as Iron Starvation Induced Protein and flavodoxin, were highly up-regulated. At phase II, down-regulation of numerous iron-containing proteins was detected in parallel to reduction in growth rate, chlorophyll content, photosynthetic activity, respiration rate, and antioxidant capacity. Intriguingly, while application of oxidative stress to phase I and II iron-limited cells similarly oxidized the reduced glutathione (GSH) pool, phase II iron limitation exhibited transient resistance to oxidative stress, despite the down regulation of many antioxidant proteins. By comparing proteomic profiles of P. tricornutum under iron limitation and metatranscriptomic data of an iron enrichment experiment conducted in the Pacific Ocean, we propose that iron-limited cells in the natural environment resemble the phase II metabolic state. These results provide insights into the trade-off between optimal growth rate and susceptibility to oxidative stress in the response of diatoms to iron quota in the marine environment.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures

References
-
- Andrews SC, Arosio P, Bottke W, Briat JF, von Darl M, Harrison PM, Laulhère JP, Levi S, Lobreaux S, Yewdall SJ (1992) Structure, function, and evolution of ferritins. J Inorg Biochem 47: 161–174 - PubMed
-
- Armbrust EV. (2009) The life of diatoms in the world’s oceans. Nature 459: 185–192 - PubMed
-
- Arrigo KR, Perovich DK, Pickart RS, Brown ZW, van Dijken GL, Lowry KE, Mills MM, Palmer MA, Balch WM, Bahr F, et al. (2012) Massive phytoplankton blooms under Arctic sea ice. Science 336: 1408. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

