Preclinical Profile and Clinical Efficacy of a Novel Hepatitis C Virus NS5A Inhibitor, EDP-239
- PMID: 27503640
- PMCID: PMC5038307
- DOI: 10.1128/AAC.00808-16
Preclinical Profile and Clinical Efficacy of a Novel Hepatitis C Virus NS5A Inhibitor, EDP-239
Abstract
EDP-239, a novel hepatitis C virus (HCV) inhibitor targeting nonstructural protein 5A (NS5A), has been investigated in vitro and in vivo EDP-239 is a potent, selective inhibitor with potency at picomolar to nanomolar concentrations against HCV genotypes 1 through 6. In the presence of human serum, the potency of EDP-239 was reduced by less than 4-fold. EDP-239 is additive to synergistic with other direct-acting antivirals (DAAs) or host-targeted antivirals (HTAs) in blocking HCV replication and suppresses the selection of resistance in vitro Furthermore, EDP-239 retains potency against known DAA- or HTA-resistant variants, with half-maximal effective concentrations (EC50s) equivalent to those for the wild type. In a phase I, single-ascending-dose, placebo-controlled clinical trial, EDP-239 demonstrated excellent pharmacokinetic properties that supported once daily dosing. A single 100-mg dose of EDP-239 resulted in reductions in HCV genotype 1a viral RNA of >3 log10 IU/ml within the first 48 h after dosing and reductions in genotype 1b viral RNA of >4-log10 IU/ml within 96 h. (This study has been registered at ClinicalTrials.gov under identifier NCT01856426.).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
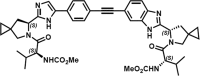




References
-
- . 1999. Hepatitis C—global prevalence (update). Wkly Epidemiol Rec 74:425–427. - PubMed
-
- . 2016. Hepatitis C fact sheet no. 164. World Health Organization, Geneva, Switzerland.
-
- Lindenbach BD, Rice CM. 2001. Flaviviridae: the viruses and their replication, p 991–1041. In Knipe DM, Howley PM (ed), Fields virology, 4th ed, vol 1 Lippincott-Raven Publishers, Philadelphia, PA.
-
- Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, Halfon P, Inchauspe G, Kuiken C, Maertens G, Mizokami M, Murphy DG, Okamoto H, Pawlotsky JM, Penin F, Sablon E, Shin IT, Stuyver LJ, Thiel HJ, Viazov S, Weiner AJ, Widell A. 2005. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 42:962–973. doi:10.1002/hep.20819. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

