StemCell Keep™ Is Effective for Cryopreservation of Human Embryonic Stem Cells by Vitrification
- PMID: 27503846
- PMCID: PMC5657710
- DOI: 10.3727/096368916X692654
StemCell Keep™ Is Effective for Cryopreservation of Human Embryonic Stem Cells by Vitrification
Abstract
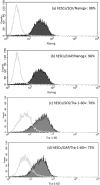
Safe and stable cryopreservation is critical for research involving human embryonic stem cells (hESCs). Dimethyl sulfoxide (DMSO) is a popular cryoprotective agent; however, its cytotoxicity cannot be ignored. Thus, there is a need for an alternate cryoprotectant. We reported previously that a novel cryopreservation reagent, StemCell Keep™ (SCK), was effective for cryopreserving human induced pluripotent stem cells (hiPSCs) by vitrification. Because hESCs and hiPSCs are not identical, the current study examined the use of SCK on hESCs. hESCs cryopreserved with SCK were thawed and cultured on SNL 76/7 cells, which were derived from a mouse fibroblast STO cell line transformed with neomycin resistance and murine LIF genes. After cryopreservation, cultured hESCs were assessed for their attachment ability and characterized by alkaline phosphatase (AP) and immunocytochemical (ICC) staining, fluorescence-activated cell sorting (FACS), reverse transcription polymerase chain reaction (RT-PCR), and karyotyping. The proliferation of SCK-cryopreserved hESCs cultured on SNL cells, or in feeder-free conditions, was higher than that of cells preserved in a solution of 2 M DMSO, 1 M acetamide, and 3 M propylene glycol (DAP). The cell number with SCK-cryopreserved hESCs was about twice that of hESCs cryopreserved in DAP. The pluripotency of SCK-cryopreserved hESCs was similar to that of DAP-cryopreserved hESCs based on AP staining. Data from ICC, FACS, and RT-PCR analyses showed that stem cell markers were continually expressed on SCK-cryopreserved hESCs. The teratoma assay showed that SCK-cryopreserved hESCs differentiated into three germ layers. Furthermore, SCK-cryopreserved hESCs had normal karyotypes. These data indicate that SCK was effective for cryopreservation of hESCs by vitrification.
Figures








References
-
- Adler S.; Pellizzer C.; Paparella M.; Hartung T.; Bremer S. The effects of solvents on embryonic stem cell differentiation. Toxicol. In Vitro 20: 265–271; 2006. - PubMed
-
- Baharvand H.; Salekdeh G. H.; Taei A.; Mollamohammadi S. An efficient and easy-to-use cryopreservation protocol for human ES and iPS cells. Nat. Protoc. 5: 588–594; 2010. - PubMed
-
- Beier A. F.; Schulz J. C.; Zimmermann H. Cryopreservation with a twist—Towards a sterile, serum-free surface-based vitrification of hESCs. Cryobiology 66: 8–16; 2013. - PubMed
-
- Chen H. H.; Chuang C. Y.; Lee W. C.; Huang H. P.; Wu H. C.; Ho H. N.; Chen Y. J.; Kuoet H. C. Surface marker epithelial cell adhesion molecule and e-cadherin facilitate the identification and selection of induced pluripotent stem cells. Stem Cell Rev. Rep. 7: 722–735; 2011. - PubMed
-
- Fujioka T.; Yasuchika K.; Nakamura Y.; Nakatsuji N.; Suemori F. A simple and efficient cryopreservation method for primate embryonic stem cells. Int. J. Dev. Biol. 48: 1149–1154; 2004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

