Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology
- PMID: 27503873
- PMCID: PMC5024607
- DOI: 10.1073/pnas.1600311113
Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology
Abstract
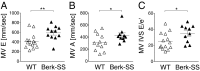
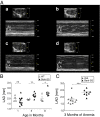
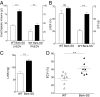
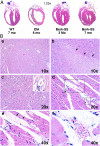
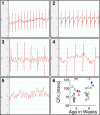
Cardiopulmonary complications are the leading cause of mortality in sickle cell anemia (SCA). Elevated tricuspid regurgitant jet velocity, pulmonary hypertension, diastolic, and autonomic dysfunction have all been described, but a unifying pathophysiology and mechanism explaining the poor prognosis and propensity to sudden death has been elusive. Herein, SCA mice underwent a longitudinal comprehensive cardiac analysis, combining state-of-the-art cardiac imaging with electrocardiography, histopathology, and molecular analysis to determine the basis of cardiac dysfunction. We show that in SCA mice, anemia-induced hyperdynamic physiology was gradually superimposed with restrictive physiology, characterized by progressive left atrial enlargement and diastolic dysfunction with preserved systolic function. This phenomenon was absent in WT mice with experimentally induced chronic anemia of similar degree and duration. Restrictive physiology was associated with microscopic cardiomyocyte loss and secondary fibrosis detectable as increased extracellular volume by cardiac-MRI. Ultrastructural mitochondrial changes were consistent with severe chronic hypoxia/ischemia and sarcomere diastolic-length was shortened. Transcriptome analysis revealed up-regulation of genes involving angiogenesis, extracellular-matrix, circadian-rhythm, oxidative stress, and hypoxia, whereas ion-channel transport and cardiac conduction were down-regulated. Indeed, progressive corrected QT prolongation, arrhythmias, and ischemic changes were noted in SCA mice before sudden death. Sudden cardiac death is common in humans with restrictive cardiomyopathies and long QT syndromes. Our findings may thus provide a unifying cardiac pathophysiology that explains the reported cardiac abnormalities and sudden death seen in humans with SCA.
Keywords: arrhythmias; cardiomyopathy; restrictive physiology; sickle cell anemia; sudden death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
The heart in sickle cell disease, a model for heart failure with preserved ejection fraction.Proc Natl Acad Sci U S A. 2016 Aug 30;113(35):9670-2. doi: 10.1073/pnas.1611899113. Epub 2016 Aug 10. Proc Natl Acad Sci U S A. 2016. PMID: 27512036 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

