Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells
- PMID: 27503932
- PMCID: PMC5050142
- DOI: 10.1158/0008-5472.CAN-16-0839
Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells
Abstract
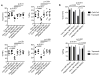
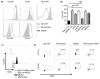
Human cytomegalovirus (CMV)-induced adaptive natural killer (NK) cells display distinct phenotypic and functional characteristics, including properties of immune memory. We hypothesized that these cells may be more resistant to suppression mediated by immunoregulatory cell subsets, making them attractive for use in cancer therapy. Here we report that relative to conventional NK cells, adaptive NK cells express lower levels of the inhibitory receptor T-cell Ig and ITIM domain (TIGIT), which results in resistance to immune suppression mediated by myeloid-derived suppressor cells (MDSC), as derived from cytokine induction in normal blood or patients with myelodysplastic syndrome. In contrast, conventional NK cells were potently suppressed by MDSCs, an effect abrogated completely by TIGIT blockade. Mechanistically, TIGIT signaling in NK cells after MDSC coculture led to a decrease in the phosphorylation of ZAP70/Syk and ERK1/2. These effects were reversed by blocking TIGIT on NK cells or by inhibiting production of reactive oxygen species (ROS) by MDSCs, the latter of which upregulated the TIGIT ligand CD155 on MDSCs. Accordingly, the blunted cytotoxicity of NK cells cocultured with MDSCs against tumor cells could be reversed by blocking TIGIT or ROS production. Overall, our results show how adaptive NK cells arising in response to CMV infection can escape MDSC-mediated suppression, and defined TIGIT antagonists as a novel type of checkpoint inhibitor to enhance NK-cell-mediated responses against cancer and infection. Cancer Res; 76(19); 5696-706. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Dr. JS Miller serves on the Scientific Advisory Board of Celgene, Fate Therapeutics and Oxis Biotech. Dr. BR Blazar declares a financial conflict with Tmunity and Kadmon Corp. Dr. Miller’s and Blazar’s relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest polices. None of these relationships had any role in this research, which was funded by the NIH. The other authors have no conflict of interest to declare.
Figures





References
-
- Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. European journal of immunology. 1975;5(2):117–21. - PubMed
-
- Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity International journal of cancer. Journal international du cancer. 1975;16(2):216–29. - PubMed
-
- Narni-Mancinelli E, Vivier E, Kerdiles YM. The ‘T-cell-ness’ of NK cells: unexpected similarities between NK cells and T cells. International immunology. 2011;23(7):427–31. - PubMed
-
- Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nature reviews Immunology. 2004;4(3):190–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

