Embracing change: striated-for-smooth muscle replacement in esophagus development
- PMID: 27504178
- PMCID: PMC4976477
- DOI: 10.1186/s13395-016-0099-1
Embracing change: striated-for-smooth muscle replacement in esophagus development
Abstract
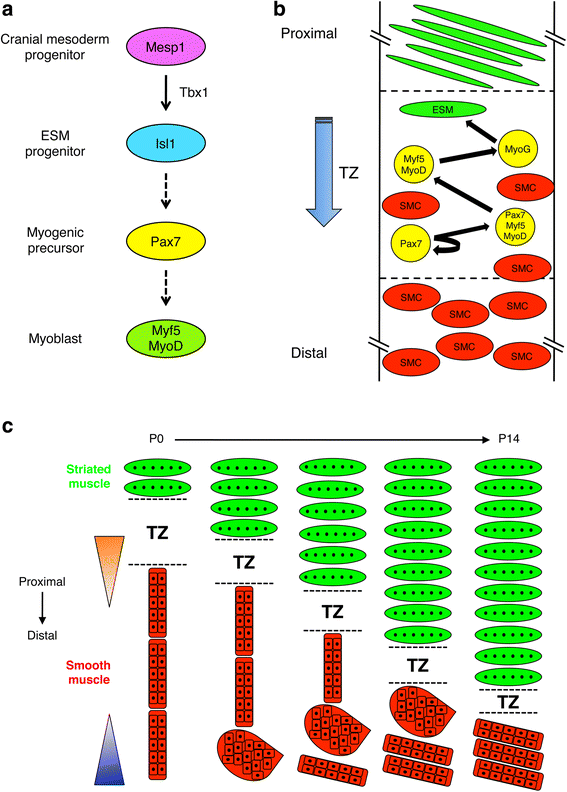
The esophagus functions to transport food from the oropharyngeal region to the stomach via waves of peristalsis and transient relaxation of the lower esophageal sphincter. The gastrointestinal tract, including the esophagus, is ensheathed by the muscularis externa (ME). However, while the ME of the gastrointestinal tract distal to the esophagus is exclusively smooth muscle, the esophageal ME of many vertebrate species comprises a variable amount of striated muscle. The esophageal ME is initially composed only of smooth muscle, but its developmental maturation involves proximal-to-distal replacement of smooth muscle with striated muscle. This fascinating phenomenon raises two important questions: what is the developmental origin of the striated muscle precursor cells, and what are the cellular and morphogenetic mechanisms underlying the process? Studies addressing these questions have provided controversial answers. In this review, we discuss the development of ideas in this area and recent work that has shed light on these issues. A working model has emerged that should permit deeper understanding of the role of ME development and maturation in esophageal disorders and in the functional and evolutionary underpinnings of the variable degree of esophageal striated myogenesis in vertebrate species.
Figures
Similar articles
-
Striated-for-smooth muscle replacement in the developing mouse esophagus.Histol Histopathol. 2019 May;34(5):457-467. doi: 10.14670/HH-18-087. Epub 2019 Jan 30. Histol Histopathol. 2019. PMID: 30698269 Review.
-
Smooth muscle persists in the muscularis externa of developing and adult mouse esophagus.J Muscle Res Cell Motil. 2007;28(2-3):153-65. doi: 10.1007/s10974-007-9112-y. Epub 2007 Jul 19. J Muscle Res Cell Motil. 2007. PMID: 17638088
-
Morphology of the developing muscularis externa in the mouse esophagus.Dis Esophagus. 2012 Jan;25(1):10-6. doi: 10.1111/j.1442-2050.2011.01208.x. Epub 2011 May 19. Dis Esophagus. 2012. PMID: 21595780
-
Ultrastructural analysis of the smooth-to-striated transition zone in the developing mouse esophagus: emphasis on apoptosis of smooth and origin and differentiation of striated muscle cells.Dev Dyn. 2005 Jul;233(3):964-82. doi: 10.1002/dvdy.20436. Dev Dyn. 2005. PMID: 15918172
-
Physiology of esophageal motor function.Gastroenterol Clin North Am. 1989 Jun;18(2):179-94. Gastroenterol Clin North Am. 1989. PMID: 2668166 Review.
Cited by
-
Histology and Ultrastructure of the Esophagus in European Beaver (Castor fiber) Displays Features Adapted to Seasonal Changes in Diet.Animals (Basel). 2023 Feb 11;13(4):635. doi: 10.3390/ani13040635. Animals (Basel). 2023. PMID: 36830422 Free PMC article.
-
Enteric co-innervation of striated muscle in the esophagus: still enigmatic?Histochem Cell Biol. 2016 Dec;146(6):721-735. doi: 10.1007/s00418-016-1500-1. Epub 2016 Sep 28. Histochem Cell Biol. 2016. PMID: 27678007 Review.
-
Tissue engineering of the gastroesophageal junction.J Tissue Eng Regen Med. 2020 Jun;14(6):855-868. doi: 10.1002/term.3045. Epub 2020 Apr 23. J Tissue Eng Regen Med. 2020. PMID: 32304170 Free PMC article. Review.
-
Transcriptome Dynamics in the Developing Larynx, Trachea, and Esophagus.Front Cell Dev Biol. 2022 Jul 22;10:942622. doi: 10.3389/fcell.2022.942622. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35938172 Free PMC article.
-
Lgr5 marks stem/progenitor cells contributing to epithelial and muscle development in the mouse esophagus.Nat Commun. 2024 Aug 21;15(1):7145. doi: 10.1038/s41467-024-51559-4. Nat Commun. 2024. PMID: 39164270 Free PMC article.
References
-
- Mittal RK, Goyal RK. Sphincter mechanisms at the lower end of the esophagus. GI Motility Online. 2006. doi:10.1038/gimo14: http://www.nature.com/gimo/contents/pt1/full/gimo14.html#relatedcontent. Accessed 16 May 2006.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

