Towards the development of an enzyme replacement therapy for the metabolic disorder propionic acidemia
- PMID: 27504265
- PMCID: PMC4968140
- DOI: 10.1016/j.ymgmr.2016.06.009
Towards the development of an enzyme replacement therapy for the metabolic disorder propionic acidemia
Abstract
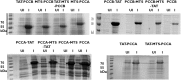
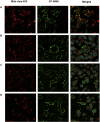
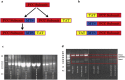
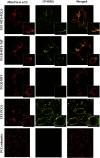
Propionic acidemia (PA) is a life-threatening disease caused by the deficiency of a mitochondrial biotin-dependent enzyme known as propionyl coenzyme-A carboxylase (PCC). This enzyme is responsible for degrading the metabolic intermediate, propionyl coenzyme-A (PP-CoA), derived from multiple metabolic pathways. Currently, except for drastic surgical and dietary intervention that can only provide partial symptomatic relief, no other form of therapeutic option is available for this genetic disorder. Here, we examine a novel approach in protein delivery by specifically targeting and localizing our protein candidate of interest into the mitochondrial matrix of the cells. In order to test this concept of delivery, we have utilized cell penetrating peptides (CPPs) and mitochondria targeting sequences (MTS) to form specific fusion PCC protein, capable of translocating and localizing across cell membranes. In vitro delivery of our candidate fusion proteins, evaluated by confocal images and enzymatic activity assay, indicated effectiveness of this strategy. Therefore, it holds immense potential in creating a new paradigm in site-specific protein delivery and enzyme replacement therapeutic for PA.
Keywords: CPPs, cell penetrating peptides; CoA, coenzyme-A; ERT, enzyme replacement therapy; Enzyme replacement therapy; His-tag, six histidines tag; LAD, lipoamine dehydrogenase; MPP, mitochondrial processing peptidase; MTS, mitochondria targeting sequences; Mitochondrial targeting sequences; PA, propionic acidemia; PCC, propionyl coenzyme-A carboxylase; PCCA, PCCα subunit; PCCB, PCCβ subunit; PP-CoA, propionyl coenzyme-A; Propionic acidemia; Propionyl coenzyme-A carboxylase; Protein transduction domains; UPLC-MS/MS, ultra performance liquid chromatography tandem mass spectrometry.
Figures



Similar articles
-
Pathophysiological mechanisms of complications associated with propionic acidemia.Pharmacol Ther. 2023 Sep;249:108501. doi: 10.1016/j.pharmthera.2023.108501. Epub 2023 Jul 22. Pharmacol Ther. 2023. PMID: 37482098 Free PMC article. Review.
-
Propionyl-CoA carboxylase - A review.Mol Genet Metab. 2017 Dec;122(4):145-152. doi: 10.1016/j.ymgme.2017.10.002. Epub 2017 Oct 7. Mol Genet Metab. 2017. PMID: 29033250 Free PMC article. Review.
-
Propionyl-CoA carboxylase pcca-1 and pccb-1 gene deletions in Caenorhabditis elegans globally impair mitochondrial energy metabolism.J Inherit Metab Dis. 2018 Mar;41(2):157-168. doi: 10.1007/s10545-017-0111-x. Epub 2017 Nov 20. J Inherit Metab Dis. 2018. PMID: 29159707 Free PMC article.
-
Determination of methylmalonyl coenzyme A by ultra high-performance liquid chromatography tandem mass spectrometry for measuring propionyl coenzyme A carboxylase activity in patients with propionic acidemia.J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Mar 1;1046:195-199. doi: 10.1016/j.jchromb.2017.02.003. Epub 2017 Feb 6. J Chromatogr B Analyt Technol Biomed Life Sci. 2017. PMID: 28189105
-
Metabolic perturbations mediated by propionyl-CoA accumulation in organs of mouse model of propionic acidemia.Mol Genet Metab. 2021 Nov;134(3):257-266. doi: 10.1016/j.ymgme.2021.09.009. Epub 2021 Oct 4. Mol Genet Metab. 2021. PMID: 34635437
Cited by
-
Pathophysiological mechanisms of complications associated with propionic acidemia.Pharmacol Ther. 2023 Sep;249:108501. doi: 10.1016/j.pharmthera.2023.108501. Epub 2023 Jul 22. Pharmacol Ther. 2023. PMID: 37482098 Free PMC article. Review.
-
Succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism: an update on pharmacological and enzyme-replacement therapeutic strategies.J Inherit Metab Dis. 2018 Jul;41(4):699-708. doi: 10.1007/s10545-018-0153-8. Epub 2018 Feb 19. J Inherit Metab Dis. 2018. PMID: 29460030 Free PMC article. Review.
-
Import of TAT-Conjugated Propionyl Coenzyme A Carboxylase Using Models of Propionic Acidemia.Mol Cell Biol. 2018 Feb 27;38(6):e00491-17. doi: 10.1128/MCB.00491-17. Print 2018 Mar 15. Mol Cell Biol. 2018. PMID: 29378828 Free PMC article.
-
The mitochondrial carboxylase PCCA interacts with Listeria monocytogenes phospholipase PlcB to modulate bacterial survival.Appl Environ Microbiol. 2024 Jun 18;90(6):e0213523. doi: 10.1128/aem.02135-23. Epub 2024 May 10. Appl Environ Microbiol. 2024. PMID: 38727222 Free PMC article.
-
Propionyl-CoA carboxylase - A review.Mol Genet Metab. 2017 Dec;122(4):145-152. doi: 10.1016/j.ymgme.2017.10.002. Epub 2017 Oct 7. Mol Genet Metab. 2017. PMID: 29033250 Free PMC article. Review.
References
-
- Amalfitano A., Bengur A.R., Morse R.P., Majure J.M., Case L.E., Veerling D.L., Mackey J., Kishnani P., Smith W., McVie-Wylie A., Sullivan J.A., Hoganson G.E., Phillips J.A., 3rd, Schaefer G.B., Charrow J., Ware R.E., Bossen E.H., Chen Y.T. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet. Med. 2001;3(2):132–138. - PubMed
-
- Barton N.W., Brady R.O., Dambrosia J.M., Di Bisceglie A.M., Doppelt S.H., Hill S.C., Mankin H.J., Murray G.J., Parker R.I., Argoff C.E. Replacement therapy for inherited enzyme deficiency — macrophage-targeted glucocerebrosidase for Gaucher's disease. N. Engl. J. Med. 1991;324(21):1464–1470. - PubMed
-
- Becker-Hapak M., McAllister S.S., Dowdy S.F. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24(3):247–256. - PubMed
-
- Chacinska A., van der Laan M., Mehnert C.S., Guiard B., Mick D.U., Hutu D.P., Truscott K.N., Wiedemann N., Meisinger C., Pfanner N., Rehling P. Distinct forms of mitochondrial TOM-TIM supercomplexes define signal-dependent states of preprotein sorting. Mol. Cell. Biol. 2010;30(1):307–318. - PMC - PubMed
-
- Chugh A., Eudes F., Shim Y.S. Cell-penetrating peptides: nanocarrier for macromolecule delivery in living cells. IUBMB Life. 2010;62(3):183–193. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

