Towards the development of an enzyme replacement therapy for the metabolic disorder propionic acidemia
- PMID: 27504265
- PMCID: PMC4968140
- DOI: 10.1016/j.ymgmr.2016.06.009
Towards the development of an enzyme replacement therapy for the metabolic disorder propionic acidemia
Abstract
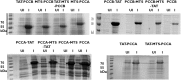
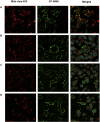
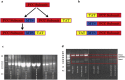
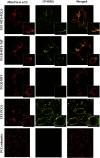
Propionic acidemia (PA) is a life-threatening disease caused by the deficiency of a mitochondrial biotin-dependent enzyme known as propionyl coenzyme-A carboxylase (PCC). This enzyme is responsible for degrading the metabolic intermediate, propionyl coenzyme-A (PP-CoA), derived from multiple metabolic pathways. Currently, except for drastic surgical and dietary intervention that can only provide partial symptomatic relief, no other form of therapeutic option is available for this genetic disorder. Here, we examine a novel approach in protein delivery by specifically targeting and localizing our protein candidate of interest into the mitochondrial matrix of the cells. In order to test this concept of delivery, we have utilized cell penetrating peptides (CPPs) and mitochondria targeting sequences (MTS) to form specific fusion PCC protein, capable of translocating and localizing across cell membranes. In vitro delivery of our candidate fusion proteins, evaluated by confocal images and enzymatic activity assay, indicated effectiveness of this strategy. Therefore, it holds immense potential in creating a new paradigm in site-specific protein delivery and enzyme replacement therapeutic for PA.
Keywords: CPPs, cell penetrating peptides; CoA, coenzyme-A; ERT, enzyme replacement therapy; Enzyme replacement therapy; His-tag, six histidines tag; LAD, lipoamine dehydrogenase; MPP, mitochondrial processing peptidase; MTS, mitochondria targeting sequences; Mitochondrial targeting sequences; PA, propionic acidemia; PCC, propionyl coenzyme-A carboxylase; PCCA, PCCα subunit; PCCB, PCCβ subunit; PP-CoA, propionyl coenzyme-A; Propionic acidemia; Propionyl coenzyme-A carboxylase; Protein transduction domains; UPLC-MS/MS, ultra performance liquid chromatography tandem mass spectrometry.
Figures



References
-
- Amalfitano A., Bengur A.R., Morse R.P., Majure J.M., Case L.E., Veerling D.L., Mackey J., Kishnani P., Smith W., McVie-Wylie A., Sullivan J.A., Hoganson G.E., Phillips J.A., 3rd, Schaefer G.B., Charrow J., Ware R.E., Bossen E.H., Chen Y.T. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet. Med. 2001;3(2):132–138. - PubMed
-
- Barton N.W., Brady R.O., Dambrosia J.M., Di Bisceglie A.M., Doppelt S.H., Hill S.C., Mankin H.J., Murray G.J., Parker R.I., Argoff C.E. Replacement therapy for inherited enzyme deficiency — macrophage-targeted glucocerebrosidase for Gaucher's disease. N. Engl. J. Med. 1991;324(21):1464–1470. - PubMed
-
- Becker-Hapak M., McAllister S.S., Dowdy S.F. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24(3):247–256. - PubMed
-
- Chacinska A., van der Laan M., Mehnert C.S., Guiard B., Mick D.U., Hutu D.P., Truscott K.N., Wiedemann N., Meisinger C., Pfanner N., Rehling P. Distinct forms of mitochondrial TOM-TIM supercomplexes define signal-dependent states of preprotein sorting. Mol. Cell. Biol. 2010;30(1):307–318. - PMC - PubMed
-
- Chugh A., Eudes F., Shim Y.S. Cell-penetrating peptides: nanocarrier for macromolecule delivery in living cells. IUBMB Life. 2010;62(3):183–193. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

