Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study
- PMID: 27504914
- PMCID: PMC5215651
- DOI: 10.1111/jdv.13746
Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study
Abstract
Background: This phase 3 trial is the first to evaluate the efficacy and safety of treatment with the systemic TNF-α inhibitor, adalimumab, for Chinese patients with moderate-to-severe plaque psoriasis.
Methods: In the 12-week, double-blind, placebo-controlled Period A, patients were randomized 4 : 1 to receive adalimumab 40 mg every-other-week (following a single 80 mg dose), or placebo every-other-week. In the subsequent 12-week, open-label, Period B, all patients received adalimumab 40 mg every-other-week starting at week 13, following a single, blinded dose at week 12 of adalimumab 80 mg or matching placebo (for patients receiving placebo or adalimumab in Period A respectively). In Period A, efficacy was analysed for all randomized patients and safety for all patients receiving ≥1 dose of the study drug.
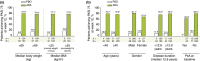
Results: For the 425 patients in this study (87 placebo; 338 adalimumab), a higher percentage randomized to adalimumab achieved the primary endpoint of ≥75% improvement from baseline in PASI score (PASI 75) at week 12: placebo 11.5% (10/87); adalimumab 77.8% (263/338; P < 0.001). Physician's Global Assessment of clear to minimal was achieved at week 12 by 14.9% placebo (13/87) and 80.5% adalimumab (272/338; P < 0.001). For patients who received adalimumab at any time during the study (All-adalimumab Population), treatment-emergent adverse events (AEs) were reported by 63.4%; the most common was upper respiratory infection (16.1%). Serious AEs were reported by 3.5% of the All-adalimumab Population, and serious infectious AEs by 1.2%, which include lung infection, pneumonia and tuberculosis [2 (0.5%) patients each]. There was one death (chronic heart failure).
Conclusion: In these Chinese patients with moderate-to-severe psoriasis, a significantly greater percentage treated with adalimumab compared with placebo achieved efficacy endpoints at week 12 and efficacy was sustained to week 24. Safety results were consistent with the known adalimumab safety profile; no new safety signals were identified in the 24 weeks of treatment.
© 2016 The Authors. Journal of European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venerology.
Figures

References
-
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology 2012; 225: 121–126. - PubMed
-
- Farley E, Menter A. Psoriasis: comorbidities and associations. G Ital Dermatol Venereol 2011; 146: 9–15. - PubMed
-
- Lebwohl M. Psoriasis. Lancet 2003; 361: 1197–1204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

