Polarized Cell Division of Chlamydia trachomatis
- PMID: 27505160
- PMCID: PMC4978491
- DOI: 10.1371/journal.ppat.1005822
Polarized Cell Division of Chlamydia trachomatis
Erratum in
-
Correction: Polarized Cell Division of Chlamydia trachomatis.PLoS Pathog. 2016 Aug 30;12(8):e1005866. doi: 10.1371/journal.ppat.1005866. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27575840 Free PMC article.
Abstract
Bacterial cell division predominantly occurs by a highly conserved process, termed binary fission, that requires the bacterial homologue of tubulin, FtsZ. Other mechanisms of bacterial cell division that are independent of FtsZ are rare. Although the obligate intracellular human pathogen Chlamydia trachomatis, the leading bacterial cause of sexually transmitted infections and trachoma, lacks FtsZ, it has been assumed to divide by binary fission. We show here that Chlamydia divides by a polarized cell division process similar to the budding process of a subset of the Planctomycetes that also lack FtsZ. Prior to cell division, the major outer-membrane protein of Chlamydia is restricted to one pole of the cell, and the nascent daughter cell emerges from this pole by an asymmetric expansion of the membrane. Components of the chlamydial cell division machinery accumulate at the site of polar growth prior to the initiation of asymmetric membrane expansion and inhibitors that disrupt the polarity of C. trachomatis prevent cell division. The polarized cell division of C. trachomatis is the result of the unipolar growth and FtsZ-independent fission of this coccoid organism. This mechanism of cell division has not been documented in other human bacterial pathogens suggesting the potential for developing Chlamydia-specific therapeutic treatments.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


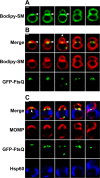
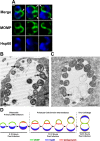
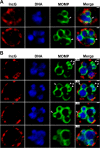

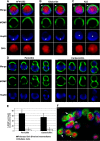
Comment in
-
Cellular microbiology: How pathogens divide.Nat Rev Microbiol. 2016 Oct;14(10):604-5. doi: 10.1038/nrmicro.2016.128. Epub 2016 Aug 30. Nat Rev Microbiol. 2016. PMID: 27573578 No abstract available.
References
-
- Wagner M, Horn M (2006) The Planctomycetes, Verrucomicrobia, Chlamydiae and sister phyla comprise a superphylum with biotechnological and medical relevance. Curr Opin Biotechnol 17: 241–249. - PubMed
-
- Abdelrahman YM, Belland RJ (2005) The chlamydial developmental cycle. FEMS Microbiol Rev 29: 949–959. - PubMed
-
- Tekniepe B, Schmidt J, Starr P (1981) Life cycle of a budding and appendaged bacterium belonging to morphotype IV of theBlastocaulis-Planctomyces group. Current Microbiology 5: 1–6.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

