Photochemical Stereocontrol Using Tandem Photoredox-Chiral Lewis Acid Catalysis
- PMID: 27505691
- PMCID: PMC5083251
- DOI: 10.1021/acs.accounts.6b00280
Photochemical Stereocontrol Using Tandem Photoredox-Chiral Lewis Acid Catalysis
Abstract
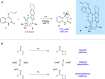
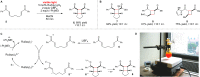
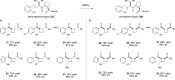
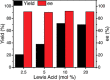
The physical, biological, and materials properties of organic compounds are determined by their three-dimensional molecular shape. The development of methods to dictate the stereochemistry of organic reactions has consequently emerged as one of the central themes of contemporary synthetic chemistry. Over the past several decades, chiral catalysts have been developed to control the enantioselectivity of almost every class of synthetically useful transformation. Photochemical reactions, however, are a conspicuous exception. Relatively few examples of highly enantioselective catalytic photoreactions have been reported to date, despite almost a century of research in this field. The development of robust strategies for photochemical enantiocontrol has thus proven to be a long-standing and surprisingly difficult challenge. For the past decade, our laboratory has been studying the application of transition metal photocatalysts to a variety of problems in synthetic organic chemistry. These efforts have recently culminated in the discovery of an effective system in which the activity of a visible light absorbing transition metal photoredox catalyst is combined with a second stereocontrolling chiral Lewis acid catalyst. This dual catalyst strategy has been applied to a diverse range of photochemical reactions; these have included highly enantioselective photocatalytic [2 + 2] cycloadditions, [3 + 2] cycloadditions, and radical conjugate addition reactions. This Account describes the development of the tandem Lewis acid photoredox catalysis strategy utilized in our laboratory. It provides an analysis of the factors that we believe to be particularly important to the success of this seemingly robust approach to photocatalytic stereocontrol. (1) The photocatalysts utilized in our systems are activated by wavelengths of visible light where the organic substrates are transparent, which minimizes the possibility of competitive racemic background photoreactions. (2) The high degree of tolerance that Ru(bpy)32+ and similar octahedral metal polypyridine complexes exhibit toward Lewis acids affords great flexibility in tuning the structure of the stereocontrolling chiral catalyst without perturbing the photoredox properties of the photocatalyst. (3) Synthetic chemists have amassed a substantial understanding of the features that are common in highly successful chiral Lewis acid catalyzed reactions, and these deep, well-validated insights are readily applied to the reactions of a variety of photogenerated intermediates. We hope that the recent success of this and similar dual catalytic systems will provide a useful foundation for the further development of powerful, stereocontrolled photochemical reactions.
Conflict of interest statement
The author declares no competing financial interest.
Figures
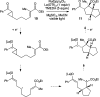
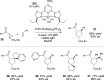

References
-
- Le Bel J. A. Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull. Soc. Chim. (Paris) 1874, 22, 337–347.
-
- van t’Hoff J. H.Die Lagerung der Atome in Raume, 2nd ed.; Vieweg: Braumschweig, 1894; p 30.
-
- Kuhn W.; Knopf E. Photochemische Erzeugung optisch aktiver Stoffe. Naturwissenschaften 1930, 18, 183.10.1007/BF01501575. - DOI
-
- Inoue Y. Asymmetric Photochemical Reactions in Solution. Chem. Rev. 1992, 92, 741–770. 10.1021/cr00013a001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

