The Enzymology of Organic Transformations: A Survey of Name Reactions in Biological Systems
- PMID: 27505692
- PMCID: PMC5477795
- DOI: 10.1002/anie.201603291
The Enzymology of Organic Transformations: A Survey of Name Reactions in Biological Systems
Abstract
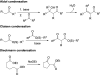
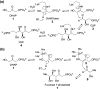
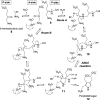
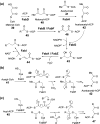
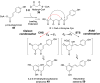
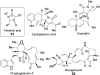
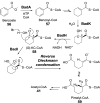
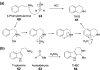
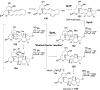
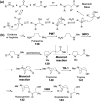
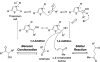
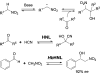
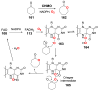
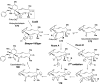
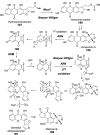
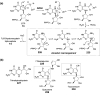
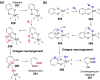
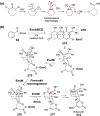
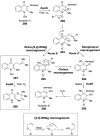
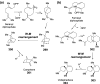
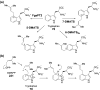
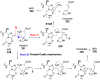
Chemical reactions that are named in honor of their true, or at least perceived, discoverers are known as "name reactions". This Review is a collection of biological representatives of named chemical reactions. Emphasis is placed on reaction types and catalytic mechanisms that showcase both the chemical diversity in natural product biosynthesis as well as the parallels with synthetic organic chemistry. An attempt has been made, whenever possible, to describe the enzymatic mechanisms of catalysis within the context of their synthetic counterparts and to discuss the mechanistic hypotheses for those reactions that are currently active areas of investigation. This Review has been categorized by reaction type, for example condensation, nucleophilic addition, reduction and oxidation, substitution, carboxylation, radical-mediated, and rearrangements, which are subdivided by name reactions.
Keywords: biosynthesis; catalysis; enzymes; name reaction; reaction mechanisms.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
























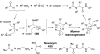




















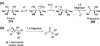

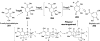


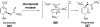



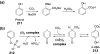

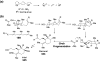

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

