Lysophosphatidic acid-induced RhoA signaling and prolonged macrophage infiltration worsens fibrosis and fatty infiltration following rotator cuff tears
- PMID: 27505847
- PMCID: PMC5502767
- DOI: 10.1002/jor.23384
Lysophosphatidic acid-induced RhoA signaling and prolonged macrophage infiltration worsens fibrosis and fatty infiltration following rotator cuff tears
Abstract
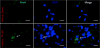
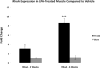
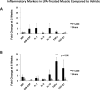
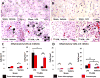
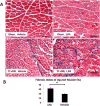
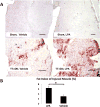
Previous studies have suggested that macrophage-mediated chronic inflammation is involved in the development of rotator cuff muscle atrophy and degeneration following massive tendon tears. Increased RhoA signaling has been reported in chronic muscle degeneration, such as muscular dystrophy. However, the role of RhoA signaling in macrophage infiltration and rotator muscle degeneration remains unknown. Using a previously established rat model of massive rotator cuff tears, we found RhoA signaling is upregulated in rotator cuff muscle following a massive tendon-nerve injury. This increase in RhoA expression is greatly potentiated by the administration of a potent RhoA activator, lysophosphatidic acid (LPA), and is accompanied by increased TNFα and TGF-β1 expression in rotator cuff muscle. Boosting RhoA signaling with LPA significantly worsened rotator cuff muscle atrophy, fibrosis, and fatty infiltration, accompanied with massive monocytic infiltration of rotator cuff muscles. Co-staining of RhoA and the tissue macrophage marker CD68 showed that CD68+ tissue macrophages are the dominant cell source of increased RhoA signaling in rotator cuff muscles after tendon tears. Taken together, our findings suggest that LPA-mediated RhoA signaling in injured muscle worsens the outcomes of atrophy, fibrosis, and fatty infiltration by increasing macrophage infiltraion in rotator cuff muscle. Clinically, inhibiting RhoA signaling may represent a future direction for developing new treatments to improve muscle quality following massive rotator cuff tears. © 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 35:1539-1547, 2017.
Keywords: LPA; atrophy; fatty infiltration; macrophage; muscle; rotator cuff tear.
© 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures


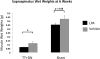
Similar articles
-
TGF-β Small Molecule Inhibitor SB431542 Reduces Rotator Cuff Muscle Fibrosis and Fatty Infiltration By Promoting Fibro/Adipogenic Progenitor Apoptosis.PLoS One. 2016 May 17;11(5):e0155486. doi: 10.1371/journal.pone.0155486. eCollection 2016. PLoS One. 2016. PMID: 27186977 Free PMC article.
-
Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear.J Shoulder Elbow Surg. 2014 Jan;23(1):99-108. doi: 10.1016/j.jse.2013.04.011. Epub 2013 Jun 20. J Shoulder Elbow Surg. 2014. PMID: 23790676 Free PMC article.
-
Beige FAPs Transplantation Improves Muscle Quality and Shoulder Function After Massive Rotator Cuff Tears.J Orthop Res. 2020 May;38(5):1159-1166. doi: 10.1002/jor.24558. Epub 2019 Dec 19. J Orthop Res. 2020. PMID: 31808573 Free PMC article.
-
Muscle degeneration in rotator cuff tears.J Shoulder Elbow Surg. 2012 Feb;21(2):164-74. doi: 10.1016/j.jse.2011.09.027. J Shoulder Elbow Surg. 2012. PMID: 22244059 Review.
-
The role of mechanobiology in progression of rotator cuff muscle atrophy and degeneration.J Orthop Res. 2018 Feb;36(2):546-556. doi: 10.1002/jor.23662. Epub 2017 Aug 11. J Orthop Res. 2018. PMID: 28755470 Free PMC article. Review.
Cited by
-
Emerging Roles of Lysophosphatidic Acid in Macrophages and Inflammatory Diseases.Int J Mol Sci. 2023 Aug 7;24(15):12524. doi: 10.3390/ijms241512524. Int J Mol Sci. 2023. PMID: 37569902 Free PMC article. Review.
-
Macrophage-specific RhoA knockout delays Wallerian degeneration after peripheral nerve injury in mice.J Neuroinflammation. 2021 Oct 15;18(1):234. doi: 10.1186/s12974-021-02292-y. J Neuroinflammation. 2021. PMID: 34654444 Free PMC article.
-
Does the Interaction between Local and Systemic Inflammation Provide a Link from Psychology and Lifestyle to Tissue Health in Musculoskeletal Conditions?Int J Mol Sci. 2021 Jul 7;22(14):7299. doi: 10.3390/ijms22147299. Int J Mol Sci. 2021. PMID: 34298917 Free PMC article. Review.
-
Single-cell transcriptome dynamics of the autotaxin-lysophosphatidic acid axis during muscle regeneration reveal proliferative effects in mesenchymal fibro-adipogenic progenitors.Front Cell Dev Biol. 2023 Feb 23;11:1017660. doi: 10.3389/fcell.2023.1017660. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36910157 Free PMC article.
-
Clinical perspectives for repairing rotator cuff injuries with multi-tissue regenerative approaches.J Orthop Translat. 2022 Aug 24;36:91-108. doi: 10.1016/j.jot.2022.06.004. eCollection 2022 Sep. J Orthop Translat. 2022. PMID: 36090820 Free PMC article. Review.
References
-
- Fehringer EV, Sun J, VanOeveren LS, et al. Full-thickness rotator cuff tear prevalence and correlation with function and co-morbidities in patients sixty-five years and older. J Shoulder Elbow Surg. 2008;17:881–885. - PubMed
-
- Gladstone JN, Bishop JY, Lo IK, et al. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–728. - PubMed
-
- Argilés JM, Busquets S, Stemmler B, et al. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

