Interaction of Staphylococcus aureus persister cells with the host when in a persister state and following awakening
- PMID: 27506163
- PMCID: PMC4979210
- DOI: 10.1038/srep31342
Interaction of Staphylococcus aureus persister cells with the host when in a persister state and following awakening
Abstract
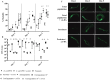
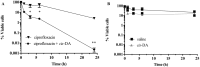
Persister cells, a tolerant cell sub-population, are commonly associated with chronic and recurrent infections. However, little is known about their ability to actually initiate or establish an infection, become virulent and cause pathogenicity within a host. Here we investigated whether Staphylococcus aureus persister cells initiate an infection and are recognized by macrophages, while in a persister cell status, and upon awakening due to exposure to cis-2-decenoic acid (cis-DA). Our results show that S. aureus persister cells are not able to initiate infections in A. thaliana and present significantly reduced virulence towards C. elegans compared to total populations. In contrast, awakened S. aureus persister cells are able to initiate infections in A. thaliana and in C. elegans albeit, with lower mortality than total population. Furthermore, exposure of S. aureus persister cells to cis-DA led to a loss of tolerance to ciprofloxacin, and an increase of the bacterial fluorescence to levels found in total population. In addition, macrophage engulfment of persister cells was significantly lower than engulfment of total population, both before and following awakening. Overall our findings indicate that upon awakening of a persister population the cells regain their ability to infect hosts despite the absence of an increased immune response.
Figures




References
-
- Bigger J. The bactericidal action of penicillin on Staphylococcus pyogenes. Irish J. Med. Sci. 19, 553–568 (1944).
-
- Keren I., Kaldalu N., Spoering A., Wang Y. & Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 230, 13–18 (2004). - PubMed
-
- Korch S. B., Henderson T. A. & Hill T. M. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol. Microbiol. 50, 1199–1213 (2003). - PubMed
-
- Fauvart M., De Groote V. N. & Michiels J. Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 60, 699–709 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

