Type I Interferons Interfere with the Capacity of mRNA Lipoplex Vaccines to Elicit Cytolytic T Cell Responses
- PMID: 27506450
- PMCID: PMC5154477
- DOI: 10.1038/mt.2016.161
Type I Interferons Interfere with the Capacity of mRNA Lipoplex Vaccines to Elicit Cytolytic T Cell Responses
Abstract
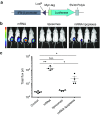
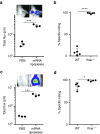
Given their high potential to evoke cytolytic T cell responses, tumor antigen-encoding messenger RNA (mRNA) vaccines are now being intensively explored as therapeutic cancer vaccines. mRNA vaccines clearly benefit from wrapping the mRNA into nano-sized carriers such as lipoplexes that protect the mRNA from degradation and increase its uptake by dendritic cells in vivo. Nevertheless, the early innate host factors that regulate the induction of cytolytic T cells to mRNA lipoplex vaccines have remained unresolved. Here, we demonstrate that mRNA lipoplexes induce a potent type I interferon (IFN) response upon subcutaneous, intradermal and intranodal injection. Regardless of the route of immunization applied, these type I IFNs interfered with the generation of potent cytolytic T cell responses. Most importantly, blocking type I IFN signaling at the site of immunization through the use of an IFNAR blocking antibody greatly enhanced the prophylactic and therapeutic antitumor efficacy of mRNA lipoplexes in the highly aggressive B16 melanoma model. As type I IFN induction appears to be inherent to the mRNA itself rather than to unique properties of the mRNA lipoplex formulation, preventing type I IFN induction and/or IFNAR signaling at the site of immunization might constitute a widely applicable strategy to improve the potency of mRNA vaccination.
Figures



References
-
- Appay, V, Douek, DC and Price, DA (2008). CD8+ T cell efficacy in vaccination and disease. Nat Med 14: 623–628. - PubMed
-
- Sahin, U, Karikó, K and Türeci, Ö (2014). mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov 13: 759–780. - PubMed
-
- Holtkamp, S, Kreiter, S, Selmi, A, Simon, P, Koslowski, M, Huber, C et al. (2006). Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 108: 4009–4017. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

