Prevention of mother-to-child transmission of hepatitis B virus: a phase III, placebo-controlled, double-blind, randomized clinical trial to assess the efficacy and safety of a short course of tenofovir disoproxil fumarate in women with hepatitis B virus e-antigen
- PMID: 27506549
- PMCID: PMC4977630
- DOI: 10.1186/s12879-016-1734-5
Prevention of mother-to-child transmission of hepatitis B virus: a phase III, placebo-controlled, double-blind, randomized clinical trial to assess the efficacy and safety of a short course of tenofovir disoproxil fumarate in women with hepatitis B virus e-antigen
Abstract
Background: Chronic hepatitis B virus (HBV) infection is complicated by cirrhosis and liver cancer. In Thailand, 6-7 % of adults are chronically infected with HBV. The risk of mother-to-child transmission (MTCT) of HBV has been estimated to be about 12 % when mothers have a high hepatitis B viral load, even if infants receive passive-active prophylaxis with HBV immunoglobulin (HBIg) and initiate the hepatitis B vaccine series at birth. We designed a study to assess the efficacy and safety of a short course of maternal tenofovir disoproxil fumarate (TDF) among women with a marker of high viral load for the prevention of MTCT of HBV.
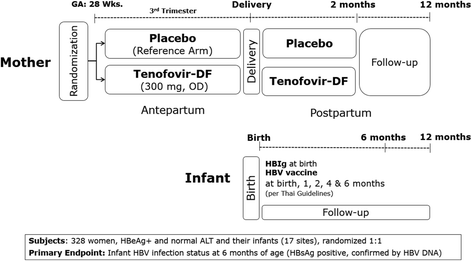
Methods: The study is a phase III, multicenter (17 sites in Thailand), placebo-controlled, double-blind, randomized 1:1, two-arm clinical trial of TDF 300 mg once daily versus placebo among pregnant women from 28 weeks' gestation through 2-month post-partum. All infants receive HBIg at birth, and a hepatitis B (HB) vaccination series according to Thai guidelines: birth, and age 1, 2, 4 and 6 months. Participant women at study entry must be age ≥18 years, hepatitis B surface antigen (HBsAg) and e-antigen (HBeAg) positive, have alanine aminotransferase (ALT) level < 30 IU/L at screening (confirmed < 60 IU/L pre-entry), negative hepatitis C serology, creatinine clearance >50 mL/min, and no history of anti-HBV antiviral treatment. The target sample size of 328 mother/infant pairs assumed 156 evaluable cases per arm to detect a ≥9 % difference in MTCT transmission (3 % experimental arm versus 12 % placebo arm) with 90 % power. Mothers and infants are followed until 12 months after delivery. The primary infant endpoint is detection of HBsAg, confirmed by detection of HBV DNA at six months of age. Secondary endpoints are maternal and infant adverse events, acute exacerbations of maternal hepatitis B disease (ALT >300 IU/L, defined as a "flare") following discontinuation of study treatment, infant HBV infection status and growth up to 12 months of age.
Discussion: The results of this randomized trial will clarify the efficacy and safety of a short course of antiviral treatment to prevent mother-to-child transmission of HBV and inform international guidelines.
Trial registration: ClinicalTrials.gov Identifier NCT01745822 .
Keywords: Hepatitis B; Hepatitis B e antigen; Hepatitis B surface antigen; Mother-to-child transmission; Pregnancy; Thailand.
References
-
- WHO. Prevention, care and treatment of persons with chronic hepatitis B infection. Guidelines [Internet]. WHO; 2015 [cited 2016 Feb 29]. Available from: http://apps.who.int/iris/bitstream/10665/154590/1/9789241549059_eng.pdf
-
- Xu W-M, Cui Y-T, Wang L, Yang H, Liang Z-Q, Li X-M, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94–103. doi: 10.1111/j.1365-2893.2008.01056.x. - DOI - PubMed
-
- Han G-R, Cao M-K, Zhao W, Jiang H-X, Wang C-M, Bai S-F, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55:1215–21. doi: 10.1016/j.jhep.2011.02.032. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical