Transcriptomic evidence for modulation of host inflammatory responses during febrile Plasmodium falciparum malaria
- PMID: 27506615
- PMCID: PMC4978957
- DOI: 10.1038/srep31291
Transcriptomic evidence for modulation of host inflammatory responses during febrile Plasmodium falciparum malaria
Abstract
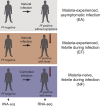
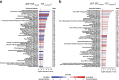
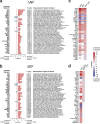
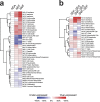
Identifying molecular predictors and mechanisms of malaria disease is important for understanding how Plasmodium falciparum malaria is controlled. Transcriptomic studies in humans have so far been limited to retrospective analysis of blood samples from clinical cases. In this prospective, proof-of-principle study, we compared whole-blood RNA-seq profiles at pre-and post-infection time points from Malian adults who were either asymptomatic (n = 5) or febrile (n = 3) during their first seasonal PCR-positive P. falciparum infection with those from malaria-naïve Dutch adults after a single controlled human malaria infection (n = 5). Our data show a graded activation of pathways downstream of pro-inflammatory cytokines, with the highest activation in malaria-naïve Dutch individuals and significantly reduced activation in malaria-experienced Malians. Newly febrile and asymptomatic infections in Malians were statistically indistinguishable except for genes activated by pro-inflammatory cytokines. The combined data provide a molecular basis for the development of a pyrogenic threshold as individuals acquire immunity to clinical malaria.
Figures

References
-
- World Health Organization. World Malaria Report 2015. (World Health Organization, 2015).
-
- Cohen S., Mc G. I. & Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature 192, 733–737 (1961). - PubMed
-
- Sabchareon A. et al.. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45, 297–308 (1991). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

