Risk of End-Stage Liver Disease in HIV-Viral Hepatitis Coinfected Persons in North America From the Early to Modern Antiretroviral Therapy Eras
- PMID: 27506682
- PMCID: PMC5064164
- DOI: 10.1093/cid/ciw531
Risk of End-Stage Liver Disease in HIV-Viral Hepatitis Coinfected Persons in North America From the Early to Modern Antiretroviral Therapy Eras
Abstract
Background: Human immunodeficiency virus (HIV)-infected patients coinfected with hepatitis B (HBV) and C (HCV) viruses are at increased risk of end-stage liver disease (ESLD). Whether modern antiretroviral therapy has reduced ESLD risk is unknown.
Methods: Twelve clinical cohorts in the United States and Canada participating in the North American AIDS Cohort Collaboration on Research and Design validated ESLD events from 1996 to 2010. ESLD incidence rates and rate ratios according to hepatitis status adjusted for age, sex, race, cohort, time-updated CD4 cell count and HIV RNA were estimated in calendar periods corresponding to major changes in antiretroviral therapy: early (1996-2000), middle (2001-2005), and modern (2006-2010) eras.
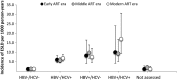
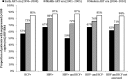
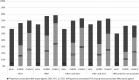
Results: Among 34 119 HIV-infected adults followed for 129 818 person-years, 380 incident ESLD outcomes occurred. ESLD incidence (per 1000 person-years) was highest in triply infected (11.57) followed by HBV- (8.72) and HCV- (6.10) coinfected vs 1.27 in HIV-monoinfected patients. Adjusted incidence rate ratios (95% confidence intervals) comparing the modern to the early antiretroviral era were 0.95 (.61-1.47) for HCV, 0.95 (.40-2.26) for HBV, and 1.52 (.46-5.02) for triply infected patients. Use of antiretrovirals dually activity against HBV increased over time. However, in the modern era, 35% of HBV-coinfected patients were not receiving tenofovir. There was little use of HCV therapy.
Conclusions: Despite increasing use of antiretrovirals, no clear reduction in ESLD risk was observed over 15 years. Treatment with direct-acting antivirals for HCV and wider use of tenofovir-based regimens for HBV should be prioritized for coinfected patients.
Keywords: HIV; coinfection; end-stage liver disease; hepatitis B virus; hepatitis C virus.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures
Comment in
-
Editorial Commentary: End-Stage Liver Disease in HIV Infection: An Avoidable Burden?Clin Infect Dis. 2016 Nov 1;63(9):1168-1170. doi: 10.1093/cid/ciw537. Epub 2016 Aug 9. Clin Infect Dis. 2016. PMID: 27506684 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- Z01 CP010176/ImNIH/Intramural NIH HHS/United States
- U01 AI031834/AI/NIAID NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- U01 AI038855/AI/NIAID NIH HHS/United States
- U54 MD007587/MD/NIMHD NIH HHS/United States
- U01 AI037613/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- K01 AI071754/AI/NIAID NIH HHS/United States
- U10 EY008052/EY/NEI NIH HHS/United States
- U01 AA020790/AA/NIAAA NIH HHS/United States
- R01 DA012568/DA/NIDA NIH HHS/United States
- R01 AA016893/AA/NIAAA NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- U01 AI035042/AI/NIAID NIH HHS/United States
- U01 DA036297/DA/NIDA NIH HHS/United States
- P30 MH062246/MH/NIMH NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- U01 AI037984/AI/NIAID NIH HHS/United States
- R01 DA011602/DA/NIDA NIH HHS/United States
- K23 EY013707/EY/NEI NIH HHS/United States
- R01 CA165937/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- P30 DK026743/DK/NIDDK NIH HHS/United States
- G12 MD007583/MD/NIMHD NIH HHS/United States
- P30 AI054999/AI/NIAID NIH HHS/United States
- K24 DA000432/DA/NIDA NIH HHS/United States
- U01 DA036935/DA/NIDA NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01 AI035041/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
- UM1 AI035043/AI/NIAID NIH HHS/United States
- CIHR/Canada
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U10 EY008057/EY/NEI NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- K01 AI093197/AI/NIAID NIH HHS/United States
- U01 AI069918/AI/NIAID NIH HHS/United States
- K24 AI118591/AI/NIAID NIH HHS/United States
- K24 AI065298/AI/NIAID NIH HHS/United States
- U01 AA013566/AA/NIAAA NIH HHS/United States
- N02 CP055504/CP/NCI NIH HHS/United States
- KL2 TR002317/TR/NCATS NIH HHS/United States
- UL1 TR000083/TR/NCATS NIH HHS/United States
- P30 AI027757/AI/NIAID NIH HHS/United States
- KL2 TR000421/TR/NCATS NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- R56 AI102622/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- F31 DA037788/DA/NIDA NIH HHS/United States
- U01 AI035039/AI/NIAID NIH HHS/United States
- R01 AG053100/AG/NIA NIH HHS/United States
- U10 EY008067/EY/NEI NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- U24 AA020794/AA/NIAAA NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

