Lyme disease and relapsing fever Borrelia elongate through zones of peptidoglycan synthesis that mark division sites of daughter cells
- PMID: 27506799
- PMCID: PMC4995948
- DOI: 10.1073/pnas.1610805113
Lyme disease and relapsing fever Borrelia elongate through zones of peptidoglycan synthesis that mark division sites of daughter cells
Abstract
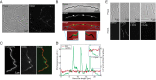
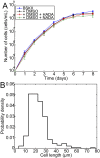
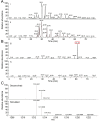
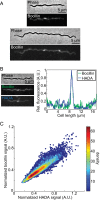
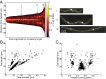
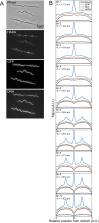
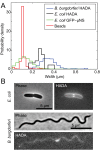
Agents that cause Lyme disease, relapsing fever, leptospirosis, and syphilis belong to the phylum Spirochaetae-a unique lineage of bacteria most known for their long, spiral morphology. Despite the relevance to human health, little is known about the most fundamental aspects of spirochete growth. Here, using quantitative microscopy to track peptidoglycan cell-wall synthesis, we found that the Lyme disease spirochete Borrelia burgdorferi displays a complex pattern of growth. B. burgdorferi elongates from discrete zones that are both spatially and temporally regulated. In addition, some peptidoglycan incorporation occurs along the cell body, with the notable exception of a large region at the poles. Newborn cells inherit a highly active zone of peptidoglycan synthesis at midcell that contributes to elongation for most of the cell cycle. Concomitant with the initiation of nucleoid separation and cell constriction, second and third zones of elongation are established at the 1/4 and 3/4 cellular positions, marking future sites of division for the subsequent generation. Positioning of elongation zones along the cell is robust to cell length variations and is relatively precise over long distances (>30 µm), suggesting that cells ‟sense" relative, as opposed to absolute, cell length to establish zones of peptidoglycan synthesis. The transition from one to three zones of peptidoglycan growth during the cell cycle is also observed in relapsing fever Borrelia. However, this mode of growth does not extend to representative species from other spirochetal genera, suggesting that this distinctive growth mode represents an evolutionary divide in the spirochete phylum.
Keywords: Borrelia burgdorferi; Lyme disease; peptidoglycan; relapsing fever; spirochetes.
Conflict of interest statement
The authors declare no conflict of interest. See QnAs on page 9129.
Figures










References
-
- Centers for Disease Control and Prevention 2013 CDC provides estimate of Americans diagnosed with Lyme disease each year. Available at www.cdc.gov/media/releases/2013/p0819-lyme-disease.html. Accessed February 17, 2016.
-
- Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2015;29(2):187–210. - PubMed
-
- Benach JL, et al. In: Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Samuels DS, Radolf JD, editors. Caister Academic; Norfolk, UK: 2010. pp. 7–442.
-
- Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32(2):149–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

