Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study)
- PMID: 27507234
- PMCID: PMC4985870
- DOI: 10.1136/bmjopen-2016-011857
Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study)
Abstract
Objective: To describe the impact of omalizumab on asthma management in patients treated as part of normal clinical practice in the UK National Health Service (NHS).
Design: A non-interventional, mixed methodology study, combining retrospective and prospective data collection for 12 months pre-omalizumab and post-omalizumab initiation, respectively.
Setting: Data were collected in 22 UK NHS centres, including specialist centres and district general hospitals in the UK.
Participants: 258 adult patients (aged ≥16 years; 65% women) with severe persistent allergic asthma treated with omalizumab were recruited, of whom 218 (84.5%) completed the study.
Primary and secondary outcome measures: The primary outcome measure was change in mean daily dose of oral corticosteroids (OCS) between the 12-month pre-omalizumab and post-omalizumab initiation periods. A priori secondary outcome measures included response to treatment, changes in OCS dosing, asthma exacerbations, lung function, employment/education, patient-reported outcomes and hospital resource utilisation.
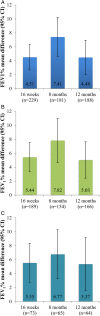
Results: The response rate to omalizumab at 16 weeks was 82.4%. Comparing pre-omalizumab and post-omalizumab periods, the mean (95% CIs) daily dose of OCS decreased by 1.61 (-2.41 to -0.80) mg/patient/day (p<0.001) and hospital exacerbations decreased by 0.97 (-1.19 to -0.75) exacerbations/patient (p<0.001). Compared with baseline, lung function, assessed by percentage of forced expiratory volume in 1 s, improved by 4.5 (2.7 to 6.3)% at 16 weeks (p<0.001; maintained at 12 months) and patient quality of life (Asthma Quality of Life Questionnaire) improved by 1.38 (1.18 to 1.58) points at 16 weeks (p<0.001, maintained at 12 months). 21/162 patients with complete employment data gained employment and 6 patients lost employment in the 12-month post-omalizumab period. The mean number of A&E visits, inpatient hospitalisations, outpatient visits (excluding for omalizumab) and number of bed days/patient decreased significantly (p<0.001) in the 12-month post-omalizumab period.
Conclusions: These data support the beneficial effects of omalizumab on asthma-related outcomes, quality of life and resource utilisation in unselected patients treated in 'real-world' clinical practice.
Keywords: Severe asthma; corticosteroids; exacerbations; omalizumab.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
References
-
- Canadian Agency for Drugs and Technologies in Health. Omalizumab treatment for adults and children with allergic asthma: a review of the clinical effectiveness, cost-effectiveness, and guidelines. Ottawa: (ON: ): Canadian Agency for Drugs and Technologies in Health, 2015. http://www.ncbi.nlm.nih.gov/books/NBK280029/ (accessed 19 May 2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
