"A novel highly stable and injectable hydrogel based on a conformationally restricted ultrashort peptide"
- PMID: 27507432
- PMCID: PMC4979021
- DOI: 10.1038/srep31167
"A novel highly stable and injectable hydrogel based on a conformationally restricted ultrashort peptide"
Abstract
Nanostructures including hydrogels based on peptides containing non protein amino acids are being considered as platform for drug delivery because of their inherent biocompatibility and additional proteolytic stability. Here we describe instantaneous self-assembly of a conformationally restricted dipeptide, LeuΔPhe, containing an α,β-dehydrophenylalanine residue into a highly stable and mechanically strong hydrogel, under mild physiological aqueous conditions. The gel successfully entrapped several hydrophobic and hydrophilic drug molecules and released them in a controlled manner. LeuΔPhe was highly biocompatible and easily injectable. Administration of an antineoplastic drug entrapped in the gel in tumor bearing mice significantly controlled growth of tumors. These characteristics make LeuΔPhe an attractive candidate for further development as a delivery platform for various biomedical applications.
Figures


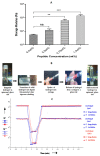
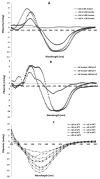
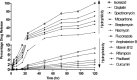
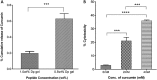

References
-
- Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nature biotechnology 21, 1171–1178 (2003). - PubMed
-
- Kobsa S. & Saltzman W. M. Bioengineering approaches to controlled protein delivery. Pediatric research 63, 513–519 (2008). - PubMed
-
- Zhang R., Tang M., Bowyer A., Eisenthal R. & Hubble J. A novel pH-and ionic-strength-sensitive carboxy methyl dextran hydrogel. Biomaterials 26, 4677–4683, (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

