Mechanistic characterization of nitrite-mediated neuroprotection after experimental cardiac arrest
- PMID: 27507435
- PMCID: PMC5247267
- DOI: 10.1111/jnc.13764
Mechanistic characterization of nitrite-mediated neuroprotection after experimental cardiac arrest
Abstract
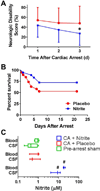
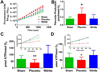
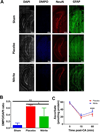
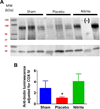
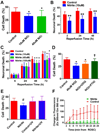
Nitrite acts as an ischemic reservoir of nitric oxide (NO) and a potent S-nitrosating agent which reduced histologic brain injury after rat asphyxial cardiac arrest (ACA). The mechanism(s) of nitrite-mediated neuroprotection remain to be defined. We hypothesized that nitrite-mediated brain mitochondrial S-nitrosation accounts for neuroprotection by reducing reperfusion reactive oxygen species (ROS) generation. Nitrite (4 μmol) or placebo was infused IV after normothermic (37°C) ACA in randomized, blinded fashion with evaluation of neurologic function, survival, brain mitochondrial function, and ROS. Blood and CSF nitrite were quantified using reductive chemiluminescence and S-nitrosation by biotin switch. Direct neuroprotection was verified in vitro after 1 and 4 h neuronal oxygen glucose deprivation measuring neuronal death with inhibition studies to examine mechanism. Mitochondrial ROS generation was quantified by live neuronal imaging using mitoSOX. Nitrite significantly reduced neurologic disability after ACA. ROS generation was reduced in brain mitochondria from nitrite- versus placebo-treated rats after ACA with congruent preservation of brain ascorbate and reduction of ROS in brain sections using immuno-spin trapping. ATP generation was maintained with nitrite up to 24 h after ACA. Nitrite rapidly entered CSF and increased brain mitochondrial S-nitrosation. Nitrite reduced in vitro mitochondrial superoxide generation and improved survival of neurons after oxygen glucose deprivation. Protection was maintained with inhibition of soluble guanylate cyclase but lost with NO scavenging and ultraviolet irradiation. Nitrite therapy results in direct neuroprotection from ACA mediated by reductions in brain mitochondrial ROS in association with protein S-nitrosation. Neuroprotection is dependent on NO and S-nitrosothiol generation, not soluble guanylate cyclase.
Keywords: cardiac arrest; cerebral ischemia; mitochondria; nitric oxide; reactive oxygen species; reperfusion injury.
© 2016 International Society for Neurochemistry.
Conflict of interest statement
Disclosures/Conflicts of Interest Dr. Dezfulian served on an ad hoc advisory board to Mallinckrodt which markets iNO.
Figures
References
-
- Basu S, Liu X, Nozari A, Rubertsson S, Miclescu A, Wiklund L. Evidence for Time-dependent Maximum Increase of Free Radical Damage and Eicosanoid Formation in the Brain as Related to Duration of Cardiac Arrest and Cardio-pulmonary Resuscitation. Free Radical Research. 2003;37:251–256. - PubMed
-
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia. New Engl J Med. 2002;346:557–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

