Cockayne syndrome: Clinical features, model systems and pathways
- PMID: 27507608
- PMCID: PMC5195851
- DOI: 10.1016/j.arr.2016.08.002
Cockayne syndrome: Clinical features, model systems and pathways
Abstract
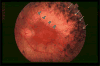
Cockayne syndrome (CS) is a disorder characterized by a variety of clinical features including cachectic dwarfism, severe neurological manifestations including microcephaly and cognitive deficits, pigmentary retinopathy, cataracts, sensorineural deafness, and ambulatory and feeding difficulties, leading to death by 12 years of age on average. It is an autosomal recessive disorder, with a prevalence of approximately 2.5 per million. There are several phenotypes (1-3) and two complementation groups (CSA and CSB), and CS overlaps with xeroderma pigmentosum (XP). It has been considered a progeria, and many of the clinical features resemble accelerated aging. As such, the study of CS affords an opportunity to better understand the underlying mechanisms of aging. The molecular basis of CS has traditionally been ascribed to defects in transcription and transcription-coupled nucleotide excision repair (TC-NER). However, recent work suggests that defects in base excision DNA repair and mitochondrial functions may also play key roles. This opens up the possibility for molecular interventions in CS, and by extrapolation, possibly in aging.
Keywords: Cockayne syndrome; Mitochondria; Neurodegeneration; Parylation; Progeria; Transcription.
Published by Elsevier B.V.
Figures


References
-
- Aamann MD, Sorensen MM, Hvitby C, Berquist BR, Muftuoglu M, Tian J, de Souza-Pinto NC, Scheibye-Knudsen M, Wilson DM, Stevnsner T, Bohr VA. Cockayne syndrome group B protein promotes mitochondrial DNA stability by supporting the DNA repair association with the mitochondrial membrane. FASEB J. 2010;24:2334–2346. doi: 10.1096/fj.09-147991. - DOI - PMC - PubMed
-
- Bartenjev I, Butina MR, Potocnik M. Rare case of Cockayne syndrome with xeroderma pigmentosum. Acta Derm Venereol. 2000;80:213–214. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

