Quiescent Bone Lining Cells Are a Major Source of Osteoblasts During Adulthood
- PMID: 27507737
- PMCID: PMC5450652
- DOI: 10.1002/stem.2474
Quiescent Bone Lining Cells Are a Major Source of Osteoblasts During Adulthood
Abstract
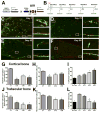
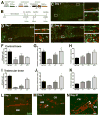
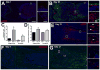
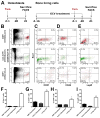
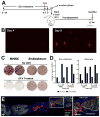
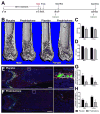
The in vivo origin of bone-producing osteoblasts is not fully defined. Skeletal stem cells, a population of mesenchymal stem cells resident in the bone marrow compartment, are thought to act as osteoprogenitors during growth and adulthood. Quiescent bone lining cells (BLCs) have been suggested as a population capable of activation into mature osteoblasts. These cells were defined by location and their morphology and studies addressing their significance have been hampered by their inaccessibility, and lack of markers that would allow for their identification and tracing. Using lineage tracing models, we have observed labeled osteoblasts at time points extending beyond the reported lifespan for this cell type, suggesting continuous reactivation of BLCs. BLCs also make a major contribution to bone formation after osteoblast ablation, which includes the ability to proliferate. In contrast, mesenchymal progenitors labeled by Gremlin1 or alpha smooth muscle actin do not contribute to bone formation in this setting. BLC activation is inhibited by glucocorticoids, which represent a well-established cause of osteoporosis. BLCs express cell surface markers characteristic of mesenchymal stem/progenitors that are largely absent in osteoblasts including Sca1 and Leptin Receptor. BLCs also show different gene expression profiles to osteoblasts, including elevated expression of Mmp13, and osteoclast regulators RANKL and macrophage colony stimulating factor, and retain osteogenic potential upon transplantation. Our findings provide evidence that bone lining cells represent a major source of osteoblasts during adulthood. Stem Cells 2016;34:2930-2942.
Keywords: Bone lining cell; Mesenchymal stem cell; Osteoblasts; Osteogenesis.
© 2016 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

