Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease
- PMID: 27507802
- PMCID: PMC5065383
- DOI: 10.1111/trf.13745
Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease
Abstract
Background: Red blood cell (RBC) hemolysis represents an intrinsic mechanism for human vascular disease. Intravascular hemolysis releases hemoglobin and other metabolites that inhibit nitric oxide signaling and drive oxidative and inflammatory stress. Although these pathways are important in disease pathogenesis, genetic and population modifiers of hemolysis, including sex, have not been established.
Study design and methods: We studied sex differences in storage or stress-induced hemolysis in RBC units from the United States and Canada in 22 inbred mouse strains and in patients with sickle cell disease (SCD) using measures of hemolysis in 315 patients who had homozygous SS hemoglobin from the Walk-PHASST cohort. A mouse model also was used to evaluate posttransfusion recovery of stored RBCs, and gonadectomy was used to determine the mechanisms related to sex hormones.
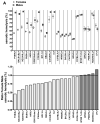
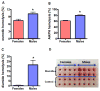
Results: An analysis of predisposition to hemolysis based on sex revealed that male RBCs consistently exhibit increased susceptibility to hemolysis compared with females in response to routine cold storage, under osmotic or oxidative stress, after transfusion in mice, and in patients with SCD. The sex difference is intrinsic to the RBC and is not mediated by plasmatic factors or female sex hormones. Importantly, orchiectomy in mice improves RBC storage stability and posttransfusion recovery, whereas testosterone repletion therapy exacerbates hemolytic response to osmotic or oxidative stress.
Conclusion: Our findings suggest that testosterone increases susceptibility to hemolysis across human diseases, suggesting that male sex may modulate clinical outcomes in blood storage and SCD and establishing a role for donor genetic variables in the viability of stored RBCs and in human hemolytic diseases.
© 2016 AABB.
Conflict of interest statement
Dr. James C. Zimring has a sponsored research agreement with Immucor, and serves on the scientific advisory board for Rubious Therapeutics. The rest of the authors declare no competing financial interests.
Figures





References
-
- Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. - PMC - PubMed
-
- Wither M, Dzieciatkowska M, Nemkov T, Strop P, D’Alessandro A, Hansen KC. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion. 2016;56:421–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

