Effects of Alteplase for Acute Stroke on the Distribution of Functional Outcomes: A Pooled Analysis of 9 Trials
- PMID: 27507856
- PMCID: PMC5024752
- DOI: 10.1161/STROKEAHA.116.013644
Effects of Alteplase for Acute Stroke on the Distribution of Functional Outcomes: A Pooled Analysis of 9 Trials
Abstract
Background: Thrombolytic therapy with intravenous alteplase within 4.5 hours of ischemic stroke onset increases the overall likelihood of an excellent outcome (no, or nondisabling, symptoms). Any improvement in functional outcome distribution has value, and herein we provide an assessment of the effect of alteplase on the distribution of the functional level by treatment delay, age, and stroke severity.
Methods: Prespecified pooled analysis of 6756 patients from 9 randomized trials comparing alteplase versus placebo/open control. Ordinal logistic regression models assessed treatment differences after adjustment for treatment delay, age, stroke severity, and relevant interaction term(s).
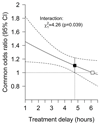
Results: Treatment with alteplase was beneficial for a delay in treatment extending to 4.5 hours after stroke onset, with a greater benefit with earlier treatment. Neither age nor stroke severity significantly influenced the slope of the relationship between benefit and time to treatment initiation. For the observed case mix of patients treated within 4.5 hours of stroke onset (mean 3 hours and 20 minutes), the net absolute benefit from alteplase (ie, the difference between those who would do better if given alteplase and those who would do worse) was 55 patients per 1000 treated (95% confidence interval, 13-91; P=0.004).
Conclusions: Treatment with intravenous alteplase initiated within 4.5 hours of stroke onset increases the chance of achieving an improved level of function for all patients across the age spectrum, including the over 80s and across all severities of stroke studied (top versus bottom fifth means: 22 versus 4); the earlier that treatment is initiated, the greater the benefit.
Keywords: United States; confidence interval; odds ratio; stroke; thrombolytic therapy.
© 2016 American Heart Association, Inc.
Conflict of interest statement
KL reports fees or expenses from American Stroke Association, Applied Clinical Intelligence, Atrium, Boehringer Ingelheim, EVER NeuroPharma, Hilicon, Nestle, Novartis, Stroke Academic Industry Roundtable, University of Lancaster; and research funding to the University of Glasgow and to the Virtual International Stroke Trials Archive from Genentech. CB, LB, and JE have not accepted fees, honoraria, or paid consultancies but are involved in clinical trials of lipid-modifying treatment funded by Merck to the University of Oxford, with the University the trial sponsor in all cases. EB is employed by Boehringer Ingelheim. SD has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Medtronic and Pfizer. GD is co-principal investigator for the EXTEND trial using alteplase and has received honoraria from Boehringer Ingelheim, Bayer, Pfizer, Sanofi and Merk Sharp & Dohme. JG has acted as a consultant for Frazer Ltd and Stryker, has received grant support from the American Heart Association, Genentech, and Behring, and has received grant support within the past 3 years from Haemonetics and Medtronics. MK reports fees and expenses from Lundbeck A/S, Mitsubishi Pharma Europe, Siemens AG. RvK reports fees from H. Lundbeck A/S, Boehringer Ingelheim, Covidien, Brainsgate, Synarc, and Penumbra, Inc. RIL has received honoraria from Boehringer Ingelheim, Covidien and Pfizer for lectures given in the past 3 years. PS has received honoraria for lectures which were paid to the department from Boehringer Ingelheim. DT reports honoraria from Boehringer Ingelheim, Bayer and Pfizer. KT has received research grant support from the Japan Agency for Medical Research and Development, and fees from Mitsubishi Tanabe Pharma. JW declares trial funding from the Medical Research Council, Efficacy and Mechanism Evaluation Programme, Stroke Association and Health Foundation. WNW is funded by a Medical Research Council Clinician Scientist Fellowship (G0902303). WH reports honoraria from Boehringer Ingelheim, Daiichi Sankyo and Bayer, receipt of an unrestricted research grant from Boehringer Ingelheim to perform the ECASS 4 EXTEND trial, and past chairmanship of the ECASS 1-3 thrombolysis trials. GH, MKa, ML and GM declare no conflicts of interest.
Figures


References
-
- Bath PMW, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, et al. for the European Stroke Organisation Outcomes Working Group Statistical Analysis of the Primary Outcome in Acute Stroke Trials. Stroke. 2012;43:1171–1178. - PubMed
-
- Emberson J, Lees KR, Lyden P, Albers G, Bluhmki E, Brott T, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;379:2352–2363. - PMC - PubMed
-
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. - PubMed
-
- Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Int Med. 1996;156:1829–1836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

