Analysis of Amyloid Precursor Protein Function in Drosophila melanogaster
- PMID: 27507933
- PMCID: PMC4960247
- DOI: 10.3389/fnmol.2016.00061
Analysis of Amyloid Precursor Protein Function in Drosophila melanogaster
Abstract
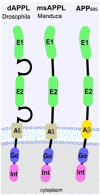
The Amyloid precursor protein (APP) has mainly been investigated in connection with its role in Alzheimer's Disease (AD) due to its cleavage resulting in the production of the Aβ peptides that accumulate in the plaques characteristic for this disease. However, APP is an evolutionary conserved protein that is not only found in humans but also in many other species, including Drosophila, suggesting an important physiological function. Besides Aβ, several other fragments are produced by the cleavage of APP; large secreted fragments derived from the N-terminus and a small intracellular C-terminal fragment. Although these fragments have received much less attention than Aβ, a picture about their function is finally emerging. In contrast to mammals, which express three APP family members, Drosophila expresses only one APP protein called APP-like or APPL. Therefore APPL functions can be studied in flies without the complication that other APP family members may have redundant functions. Flies lacking APPL are viable but show defects in neuronal outgrowth in the central and peripheral nervous system (PNS) in addition to synaptic changes. Furthermore, APPL has been connected with axonal transport functions. In the adult nervous system, APPL, and more specifically its secreted fragments, can protect neurons from degeneration. APPL cleavage also prevents glial death. Lastly, APPL was found to be involved in behavioral deficits and in regulating sleep/activity patterns. This review, will describe the role of APPL in neuronal development and maintenance and briefly touch on its emerging function in circadian rhythms while an accompanying review will focus on its role in learning and memory formation.
Keywords: Drosophila melanogaster; amyloid precursor proteins; neuronal outgrowth; neuronal survival; synaptogenesis.
Figures

References
-
- Arai H., Lee V. M., Messinger M. L., Greenberg B. D., Lowery D. E., Trojanowski J. Q. (1991). Expression patterns of beta-amyloid precursor protein (beta-APP) in neural and nonneural human tissues from Alzheimer’s disease and control subjects. Ann. Neurol. 30, 686–693. 10.1002/ana.410300509 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

