The Root Hair Specific SYP123 Regulates the Localization of Cell Wall Components and Contributes to Rizhobacterial Priming of Induced Systemic Resistance
- PMID: 27507978
- PMCID: PMC4961009
- DOI: 10.3389/fpls.2016.01081
The Root Hair Specific SYP123 Regulates the Localization of Cell Wall Components and Contributes to Rizhobacterial Priming of Induced Systemic Resistance
Abstract
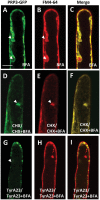
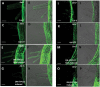
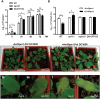
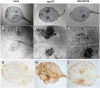
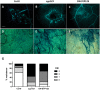
Root hairs are important for nutrient and water uptake and are also critically involved the interaction with soil inhabiting microbiota. Root hairs are tubular-shaped outgrowths that emerge from trichoblasts. This polarized elongation is maintained and regulated by a robust mechanism involving the endomembrane secretory and endocytic system. Members of the syntaxin family of SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) in plants (SYP), have been implicated in regulation of the fusion of vesicles with the target membranes in both exocytic and endocytic pathways. One member of this family, SYP123, is expressed specifically in the root hairs and accumulated in the growing tip region. This study shows evidence of the SYP123 role in polarized trafficking using knockout insertional mutant plants. We were able to observe defects in the deposition of cell wall proline rich protein PRP3 and cell wall polysaccharides. In a complementary strategy, similar results were obtained using a plant expressing a dominant negative soluble version of SYP123 (SP2 fragment) lacking the transmembrane domain. The evidence presented indicates that SYP123 is also regulating PRP3 protein distribution by recycling by endocytosis. We also present evidence that indicates that SYP123 is necessary for the response of roots to plant growth promoting rhizobacterium (PGPR) in order to trigger trigger induced systemic response (ISR). Plants with a defective SYP123 function were unable to mount a systemic acquired resistance in response to bacterial pathogen infection and ISR upon interaction with rhizobacteria. These results indicated that SYP123 was involved in the polarized localization of protein and polysaccharides in growing root hairs and that this activity also contributed to the establishment of effective plant defense responses. Root hairs represent very plastic structures were many biotic and abiotic factors can affect the number, anatomy and physiology of root hairs. Here, we presented evidence that indicates that interactions with soil PGPR could be closely regulated by signaling involving secretory and/or endocytic trafficking at the root hair tip as a quick way to response to changing environmental conditions.
Keywords: PRP3; cell wall; induced systemic resistance; plant growth promoting rhizobacterium; rhizobacteria; syntaxin; systemic acquired resistance; trafficking.
Figures

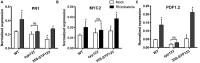
References
-
- Albersheim P., Darvill A., Roberts K., Sederoff R., Staehelin A. (2011). “Biochemistry of the cell wall molecules,” in Plant Cell Walls: From Chemistry to Biology eds Albersheim P., Darvill A., Roberts K., Serderoff R., Staehelin A. (New York, NY: Garland Science; ) 67–118.
-
- Baluška F., Salaj J., Mathur J., Braun M., Jasper F., Šamaj J., et al. (2000). Root hair formation: f-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol. 227 618–632. 10.1006/dbio.2000.9908 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

