Neurobiology of opioid dependence in creating addiction vulnerability
- PMID: 27508068
- PMCID: PMC4955026
- DOI: 10.12688/f1000research.8369.1
Neurobiology of opioid dependence in creating addiction vulnerability
Abstract
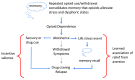
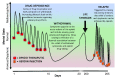
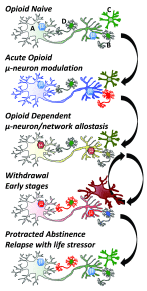
Opioid drugs are potent modulators of many physiological and psychological processes. When given acutely, they can elicit the signature responses of euphoria and analgesia that societies have coveted for centuries. Repeated, or chronic, use of opioids induces adaptive or allostatic changes that modify neuronal circuitry and create an altered normality - the "drug-dependent" state. This state, at least that exhibited by those maintained continuously on long-acting opioid drugs such as methadone or buprenorphine, is generally indistinguishable from the drug-naïve state for most overt behaviors. The consequences of the allostatic changes (cellular, circuit, and system adaptations) that accompany the drug-dependent state are revealed during drug withdrawal. Drug cessation triggers a temporally orchestrated allostatic re-establishment of neuronal systems, which is manifested as opposing physiological and psychological effects to those exhibited by acute drug intoxication. Some withdrawal symptoms, such as physical symptoms (sweating, shaking, and diarrhea) resolve within days, whilst others, such as dysphoria, insomnia, and anxiety, can linger for months, and some adaptations, such as learned associations, may be established for life. We will briefly discuss the cellular mechanisms and neural circuitry that contribute to the opioid drug-dependent state, inferring an emerging role for neuroinflammation. We will argue that opioid addictive behaviors result from a learned relationship between opioids and relief from an existing or withdrawal-induced anxiogenic and/or dysphoric state. Furthermore, a future stressful life event can recall the memory that opioid drugs alleviate negative affect (despair, sadness, and anxiety) and thereby precipitate craving, resulting in relapse. A learned association of relief of aversive states would fuel drug craving in vulnerable people living in an increasingly stressful society. We suggest that this route to addiction is contributive to the current opioid epidemic in the USA.
Keywords: Learned Associative Model; aversive states; opioid epidemic; pass-forward allostasis; withdrawal relief.
Conflict of interest statement
No competing interests were disclosed.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

