External quality assessment of dengue and chikungunya diagnostics in the Asia Pacific region, 2015
- PMID: 27508088
- PMCID: PMC4957610
- DOI: 10.5365/WPSAR.2016.7.1.002
External quality assessment of dengue and chikungunya diagnostics in the Asia Pacific region, 2015
Abstract
Objective: To conduct an external quality assessment (EQA) of dengue and chikungunya diagnostics among national-level public health laboratories in the Asia Pacific region following the first round of EQA for dengue diagnostics in 2013.
Methods: Twenty-four national-level public health laboratories performed routine diagnostic assays on a proficiency testing panel consisting of two modules. Module A contained serum samples spiked with cultured dengue virus (DENV) or chikungunya virus (CHIKV) for the detection of nucleic acid and DENV non-structural protein 1 (NS1) antigen. Module B contained human serum samples for the detection of anti-DENV antibodies.
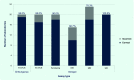
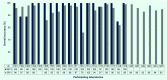
Results: Among 20 laboratories testing Module A, 17 (85%) correctly detected DENV RNA by reverse transcription polymerase chain reaction (RT-PCR), 18 (90%) correctly determined serotype and 19 (95%) correctly identified CHIKV by RT-PCR. Ten of 15 (66.7%) laboratories performing NS1 antigen assays obtained the correct results. In Module B, 18/23 (78.3%) and 20/20 (100%) of laboratories correctly detected anti-DENV IgM and IgG, respectively. Detection of acute/recent DENV infection by both molecular (RT-PCR) and serological methods (IgM) was available in 19/24 (79.2%) participating laboratories.
Discussion: Accurate laboratory testing is a critical component of dengue and chikungunya surveillance and control. This second round of EQA reveals good proficiency in molecular and serological diagnostics of these diseases in the Asia Pacific region. Further comprehensive diagnostic testing, including testing for Zika virus, should comprise future iterations of the EQA.
Figures
References
-
- Dengue Situation Update - 3 June 2014. Manila: World Health Organization Regional Office for the Western Pacific; 2014. http://www.wpro.who.int/entity/emerging_diseases/Dengue.Biweekly.03Jun20... accessed 17 December 2015.
-
- Dengue Situation Update - 13 January 2015. Manila: World Health Organization Regional Office for the Western Pacific; 2015. http://www.wpro.who.int/entity/emerging_diseases/denguebiweekly_13jan201... accessed 17 December 2015.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
