Discovery of a Series of Acridinones as Mechanism-Based Tubulin Assembly Inhibitors with Anticancer Activity
- PMID: 27508497
- PMCID: PMC4980028
- DOI: 10.1371/journal.pone.0160842
Discovery of a Series of Acridinones as Mechanism-Based Tubulin Assembly Inhibitors with Anticancer Activity
Abstract
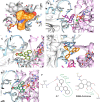
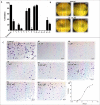
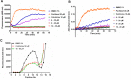
Microtubules play critical roles in vital cell processes, including cell growth, division, and migration. Microtubule-targeting small molecules are chemotherapeutic agents that are widely used in the treatment of cancer. Many of these compounds are structurally complex natural products (e.g., paclitaxel, vinblastine, and vincristine) with multiple stereogenic centers. Because of the scarcity of their natural sources and the difficulty of their partial or total synthesis, as well as problems related to their bioavailability, toxicity, and resistance, there is an urgent need for novel microtubule binding agents that are effective for treating cancer but do not have these disadvantages. In the present work, our lead discovery effort toward less structurally complex synthetic compounds led to the discovery of a series of acridinones inspired by the structure of podophyllotoxin, a natural product with important microtubule assembly inhibitory activity, as novel mechanism-based tubulin assembly inhibitors with potent anticancer properties and low toxicity. The compounds were evaluated in vitro by wound healing assays employing the metastatic and triple negative breast cancer cell line MDA-MB-231. Four compounds with IC50 values between 0.294 and 1.7 μM were identified. These compounds showed selective cytotoxicity against MDA-MB-231 and DU-145 cancer cell lines and promoted cell cycle arrest in G2/M phase and apoptosis. Consistent with molecular modeling results, the acridinones inhibited tubulin assembly in in vitro polymerization assays with IC50 values between 0.9 and 13 μM. Their binding to the colchicine-binding site of tubulin was confirmed through competitive assays.
Conflict of interest statement
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

