Human and Mouse Brown Adipose Tissue Mitochondria Have Comparable UCP1 Function
- PMID: 27508873
- PMCID: PMC5201422
- DOI: 10.1016/j.cmet.2016.07.004
Human and Mouse Brown Adipose Tissue Mitochondria Have Comparable UCP1 Function
Abstract
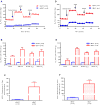
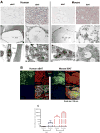
Brown adipose tissue (BAT) plays an important role in mammalian thermoregulation. The component of BAT mitochondria that permits this function is the inner membrane carrier protein uncoupling protein 1 (UCP1). To the best of our knowledge, no studies have directly quantified UCP1 function in human BAT. Further, whether human and rodent BAT have comparable thermogenic function remains unknown. We employed high-resolution respirometry to determine the respiratory capacity, coupling control, and, most importantly, UCP1 function of human supraclavicular BAT and rodent interscapular BAT. Human BAT was sensitive to the purine nucleotide GDP, providing the first direct evidence that human BAT mitochondria have thermogenically functional UCP1. Further, our data demonstrate that human and rodent BAT have similar UCP1 function per mitochondrion. These data indicate that human and rodent BAT are qualitatively similar in terms of UCP1 function.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

