Highly Selective Dopamine D3 Receptor (D3R) Antagonists and Partial Agonists Based on Eticlopride and the D3R Crystal Structure: New Leads for Opioid Dependence Treatment
- PMID: 27508895
- PMCID: PMC5001167
- DOI: 10.1021/acs.jmedchem.6b00860
Highly Selective Dopamine D3 Receptor (D3R) Antagonists and Partial Agonists Based on Eticlopride and the D3R Crystal Structure: New Leads for Opioid Dependence Treatment
Abstract
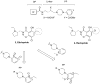
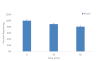
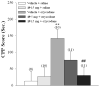
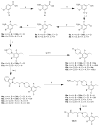
The recent and precipitous increase in opioid analgesic abuse and overdose has inspired investigation of the dopamine D3 receptor (D3R) as a target for therapeutic intervention. Metabolic instability or predicted toxicity has precluded successful translation of previously reported D3R-selective antagonists to clinical use for cocaine abuse. Herein, we report a series of novel and D3R crystal structure-guided 4-phenylpiperazines with exceptionally high D3R affinities and/or selectivities with varying efficacies. Lead compound 19 was selected based on its in vitro profile: D3R Ki = 6.84 nM, 1700-fold D3R versus D2R binding selectivity, and its metabolic stability in mouse microsomes. Compound 19 inhibited oxycodone-induced hyperlocomotion in mice and reduced oxycodone-induced locomotor sensitization. In addition, pretreatment with 19 also dose-dependently inhibited the acquisition of oxycodone-induced conditioned place preference (CPP) in rats. These findings support the D3R as a target for opioid dependence treatment and compound 19 as a new lead molecule for development.
Figures




References
-
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. - PubMed
-
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. - PubMed
-
- Pich EM, Collo G. Pharmacological targeting of dopamine D3 receptors: Possible clinical applications of selective drugs. Eur Neuropsychopharmacol. 2015;25:1437–1447. - PubMed
-
- Le Foll B, Collo G, Rabiner EA, Boileau I, Merlo Pich E, Sokoloff P. Dopamine D3 receptor ligands for drug addiction treatment: update on recent findings. Prog Brain Res. 2014;211:255–275. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

