Diversity of Human and Macaque Airway Immune Cells at Baseline and during Tuberculosis Infection
- PMID: 27509488
- PMCID: PMC5248955
- DOI: 10.1165/rcmb.2016-0122OC
Diversity of Human and Macaque Airway Immune Cells at Baseline and during Tuberculosis Infection
Abstract
Immune cells of the distal airways serve as "first responders" of host immunity to the airborne pathogen Mycobacterium tuberculosis (Mtb). Mtb infection of cynomolgus macaques recapitulates the range of human outcomes from clinically silent latent tuberculosis infection (LTBI) to active tuberculosis of various degrees of severity. To further advance the application of this model to human studies, we compared profiles of bronchoalveolar lavage (BAL) cells of humans and cynomolgus macaques before and after Mtb infection. A simple gating strategy effectively defined BAL T-cell and phagocyte populations in both species. BAL from Mtb-naive humans and macaques showed similar differential cell counts. BAL T cells of macaques were composed of fewer CD4+cells but more CD8+ and CD4+CD8+ double-positive cells than were BAL T cells of humans. The most common mononuclear phagocyte population in BAL of both species displayed coexpression of HLA-DR, CD206, CD11b, and CD11c; however, multiple phagocyte subsets displaying only some of these markers were observed as well. Macaques with LTBI displayed a marked BAL lymphocytosis that was not observed in humans with LTBI. In macaques, the prevalence of specific mononuclear phagocyte subsets in baseline BAL correlated with ultimate outcomes of Mtb infection (i.e., LTBI versus active disease). Overall, these findings demonstrate the comparability of studies of pulmonary immunity to Mtb in humans and macaques. They also indicate a previously undescribed complexity of airway mononuclear phagocyte populations that suggests further lines of investigation relevant to understanding the mechanisms of both protection from and susceptibility to the development of active tuberculosis within the lung.
Keywords: alveolar macrophages; bronchoalveolar lavage; human subjects; macaques; tuberculosis.
Figures

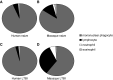


 P = 0.07, *P < 0.05, Mann–Whitney). Results for individual macaques as well as median values are displayed.
P = 0.07, *P < 0.05, Mann–Whitney). Results for individual macaques as well as median values are displayed.Similar articles
-
Pulmonary responses to pathogen-specific antigens in latent Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2016 Jan;96:158-64. doi: 10.1016/j.tube.2015.10.006. Epub 2015 Dec 8. Tuberculosis (Edinb). 2016. PMID: 26732045 Free PMC article.
-
Immune cells in bronchoalveolar lavage fluid of Ugandan adults who resist versus those who develop latent Mycobacterium tuberculosis infection.PLoS One. 2021 Apr 9;16(4):e0249477. doi: 10.1371/journal.pone.0249477. eCollection 2021. PLoS One. 2021. PMID: 33836031 Free PMC article.
-
Mucosal-activated invariant T cells do not exhibit significant lung recruitment and proliferation profiles in macaques in response to infection with Mycobacterium tuberculosis CDC1551.Tuberculosis (Edinb). 2019 May;116S:S11-S18. doi: 10.1016/j.tube.2019.04.006. Epub 2019 Apr 26. Tuberculosis (Edinb). 2019. PMID: 31072689 Free PMC article.
-
Genome wide approaches discover novel Mycobacterium tuberculosis antigens as correlates of infection, disease, immunity and targets for vaccination.Semin Immunol. 2018 Oct;39:88-101. doi: 10.1016/j.smim.2018.07.001. Epub 2018 Jul 7. Semin Immunol. 2018. PMID: 30327124 Review.
-
IL-22: An Underestimated Player in Natural Resistance to Tuberculosis?Front Immunol. 2018 Sep 25;9:2209. doi: 10.3389/fimmu.2018.02209. eCollection 2018. Front Immunol. 2018. PMID: 30319650 Free PMC article. Review.
Cited by
-
Extracellular Vesicle ASC: A Novel Mediator for Lung-Brain Axis in Preterm Brain Injury.Am J Respir Cell Mol Biol. 2024 Oct;71(4):464-480. doi: 10.1165/rcmb.2023-0402OC. Am J Respir Cell Mol Biol. 2024. PMID: 38959416 Free PMC article.
-
Immune Response to Biofilm Growing Pulmonary Pseudomonas aeruginosa Infection.Biomedicines. 2022 Aug 24;10(9):2064. doi: 10.3390/biomedicines10092064. Biomedicines. 2022. PMID: 36140163 Free PMC article. Review.
-
Absence of c-Maf and IL-10 enables Type I IFN enhancement of innate responses to low-dose LPS in alveolar macrophages.bioRxiv [Preprint]. 2024 May 26:2024.05.22.594428. doi: 10.1101/2024.05.22.594428. bioRxiv. 2024. Update in: J Immunol. 2025 Mar 1;214(3):551-564. doi: 10.1093/jimmun/vkae029. PMID: 38826239 Free PMC article. Updated. Preprint.
-
Absence of c-Maf and IL-10 enables type I IFN enhancement of innate responses to LPS in alveolar macrophages.J Immunol. 2025 Mar 1;214(3):551-564. doi: 10.1093/jimmun/vkae029. J Immunol. 2025. PMID: 40073087
-
Toll-like receptor 2bright cells identify circulating monocytes in human and non-human primates.Cytometry A. 2017 Apr;91(4):364-371. doi: 10.1002/cyto.a.23098. Epub 2017 Mar 21. Cytometry A. 2017. PMID: 28323396 Free PMC article.
References
-
- Beverley PC, Sridhar S, Lalvani A, Tchilian EZ. Harnessing local and systemic immunity for vaccines against tuberculosis. Mucosal Immunol. 2014;7:20–26. - PubMed
-
- Xing Z, Jeyanathan M, Smaill F. New approaches to TB vaccination. Chest. 2014;146:804–812. - PubMed
-
- Capuano SV, III, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–5844. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

