HCO3- Transport through Anoctamin/Transmembrane Protein ANO1/TMEM16A in Pancreatic Acinar Cells Regulates Luminal pH
- PMID: 27510033
- PMCID: PMC5034034
- DOI: 10.1074/jbc.M116.750224
HCO3- Transport through Anoctamin/Transmembrane Protein ANO1/TMEM16A in Pancreatic Acinar Cells Regulates Luminal pH
Abstract
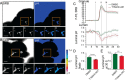
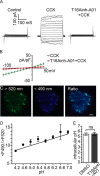
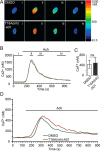
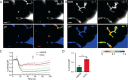
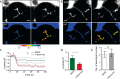
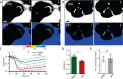
The identification of ANO1/TMEM16A as the likely calcium-dependent chloride channel of exocrine glands has led to a more detailed understanding of its biophysical properties. This includes a calcium-dependent change in channel selectivity and evidence that HCO3 (-) permeability can be significant. Here we use freshly isolated pancreatic acini that preserve the luminal structure to measure intraluminal pH and test the idea that ANO1/TMEM16A contributes to luminal pH balance. Our data show that, under physiologically relevant stimulation with 10 pm cholesystokinin, the luminal acid load that results from the exocytic fusion of zymogen granules is significantly blunted by HCO3 (-) buffer in comparison with HEPES, and that this is blocked by the specific TMEM16A inhibitor T16inh-A01. Furthermore, in a model of acute pancreatitis, we observed substantive luminal acidification and provide evidence that ANO1/TMEM16A acts to attenuate this pH shift. We conclude that ANO1/TMEM16A is a significant pathway in pancreatic acinar cells for HCO3 (-) secretion into the lumen.
Keywords: bicarbonate; epithelial cell; exocytosis; ion channel; pancreas; secretion; transporter.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Dynamic modulation of ANO1/TMEM16A HCO3(-) permeability by Ca2+/calmodulin.Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):360-5. doi: 10.1073/pnas.1211594110. Epub 2012 Dec 17. Proc Natl Acad Sci U S A. 2013. PMID: 23248295 Free PMC article.
-
Pore dilatation increases the bicarbonate permeability of CFTR, ANO1 and glycine receptor anion channels.J Physiol. 2016 Jun 1;594(11):2929-55. doi: 10.1113/JP271311. Epub 2016 Feb 2. J Physiol. 2016. PMID: 26663196 Free PMC article.
-
Slc26a6 is an apical membrane anion exchanger that drives HCO3--dependent fluid secretion in murine pancreatic acinar cells.Am J Physiol Cell Physiol. 2019 Dec 1;317(6):C1153-C1160. doi: 10.1152/ajpcell.00257.2019. Epub 2019 Sep 18. Am J Physiol Cell Physiol. 2019. PMID: 31532720 Free PMC article.
-
TMEM16A (ANO1) as a therapeutic target in cystic fibrosis.Curr Opin Pharmacol. 2022 Jun;64:102206. doi: 10.1016/j.coph.2022.102206. Epub 2022 Mar 29. Curr Opin Pharmacol. 2022. PMID: 35364521 Review.
-
Anoctamin 1 in secretory epithelia.Cell Calcium. 2014 Jun;55(6):355-61. doi: 10.1016/j.ceca.2014.02.006. Epub 2014 Feb 15. Cell Calcium. 2014. PMID: 24636668 Review.
Cited by
-
Cl- as a bona fide signaling ion.Am J Physiol Cell Physiol. 2020 Jan 1;318(1):C125-C136. doi: 10.1152/ajpcell.00354.2019. Epub 2019 Nov 6. Am J Physiol Cell Physiol. 2020. PMID: 31693396 Free PMC article. Review.
-
Human pancreatic ductal organoids with controlled polarity provide a novel ex vivo tool to study epithelial cell physiology.Cell Mol Life Sci. 2023 Jun 28;80(7):192. doi: 10.1007/s00018-023-04836-2. Cell Mol Life Sci. 2023. PMID: 37380797 Free PMC article.
-
Ion Channels Orchestrate Pancreatic Ductal Adenocarcinoma Progression and Therapy.Front Pharmacol. 2021 Jan 19;11:586599. doi: 10.3389/fphar.2020.586599. eCollection 2020. Front Pharmacol. 2021. PMID: 33841132 Free PMC article. Review.
-
Polymodal Control of TMEM16x Channels and Scramblases.Int J Mol Sci. 2022 Jan 29;23(3):1580. doi: 10.3390/ijms23031580. Int J Mol Sci. 2022. PMID: 35163502 Free PMC article. Review.
-
TMEM16A Ca2+-activated Cl- channel inhibition ameliorates acute pancreatitis via the IP3R/Ca2+/NFκB/IL-6 signaling pathway.J Adv Res. 2020 Jan 21;23:25-35. doi: 10.1016/j.jare.2020.01.006. eCollection 2020 May. J Adv Res. 2020. PMID: 32071789 Free PMC article.
References
-
- Freedman S. D., Kern H. F., and Scheele G. A. (2001) Pancreatic acinar cell dysfunction in CFTR−/− mice is associated with impairments in luminal pH and endocytosis. Gastroenterology 121, 950–957 - PubMed
-
- Dutta S. K., Russell R. M., and Iber F. L. (1979) Influence of exocrine pancreatic insufficiency on the intraluminal pH of the proximal small-intestine. Dig. Dis. Sci. 24, 529–534 - PubMed
-
- Sohma Y., Gray M. A., Imai Y., and Argent B. E. (2001) 150 mM HCO3−: how does the pancreas do it? Clues from computer modelling of the duct cell. JOP 2, 198–202 - PubMed
-
- Behrendorff N., Floetenmeyer M., Schwiening C., and Thorn P. (2010) Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology 139, 1711–1720.e1–5 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

