Phosphatidic Acid Produced by RalA-activated PLD2 Stimulates Caveolae-mediated Endocytosis and Trafficking in Endothelial Cells
- PMID: 27510034
- PMCID: PMC5034062
- DOI: 10.1074/jbc.M116.752485
Phosphatidic Acid Produced by RalA-activated PLD2 Stimulates Caveolae-mediated Endocytosis and Trafficking in Endothelial Cells
Abstract
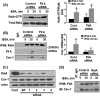
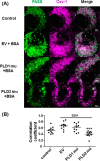
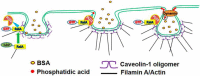
Caveolae are the primary route for internalization and transendothelial transport of macromolecules, such as insulin and albumin. Caveolae-mediated endocytosis is activated by Src-dependent caveolin-1 (Cav-1) phosphorylation and subsequent recruitment of dynamin-2 and filamin A (FilA), which facilitate vesicle fission and trafficking, respectively. Here, we tested the role of RalA and phospholipase D (PLD) signaling in the regulation of caveolae-mediated endocytosis and trafficking. The addition of albumin to human lung microvascular endothelial cells induced the activation of RalA within minutes, and siRNA-mediated down-regulation of RalA abolished fluorescent BSA uptake. Co-immunoprecipitation studies revealed that albumin induced the association between RalA, Cav-1, and FilA; however, RalA knockdown with siRNA did not affect FilA recruitment to Cav-1, suggesting that RalA was not required for FilA and Cav-1 complex formation. Rather, RalA probably facilitates caveolae-mediated endocytosis by activating downstream effectors. PLD2 was shown to be activated by RalA, and inhibition of PLD2 abolished Alexa-488-BSA uptake, indicating that phosphatidic acid (PA) generated by PLD2 may facilitate caveolae-mediated endocytosis. Furthermore, using a PA biosensor, GFP-PASS, we observed that BSA induced an increase in PA co-localization with Cav-1-RFP, which could be blocked by a dominant negative PLD2 mutant. Total internal reflection fluorescence microscopy studies of Cav-1-RFP also showed that fusion of caveolae with the basal plasma membrane was dependent on PLD2 activity. Thus, our results suggest that the small GTPase RalA plays an important role in promoting invagination and trafficking of caveolae, not by potentiating the association between Cav-1 and FilA but by stimulating PLD2-mediated generation of phosphatidic acid.
Keywords: Ras protein; caveolin; endocytosis; filamin; trafficking.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






Similar articles
-
Filamin A regulates caveolae internalization and trafficking in endothelial cells.Mol Biol Cell. 2009 Nov;20(21):4531-40. doi: 10.1091/mbc.e08-10-0997. Epub 2009 Sep 16. Mol Biol Cell. 2009. PMID: 19759182 Free PMC article.
-
Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells.J Biol Chem. 2004 Nov 12;279(46):48055-62. doi: 10.1074/jbc.M405837200. Epub 2004 Sep 2. J Biol Chem. 2004. PMID: 15345719
-
Ral isoforms are implicated in Fc gamma R-mediated phagocytosis: activation of phospholipase D by RalA.J Immunol. 2010 Sep 1;185(5):2942-50. doi: 10.4049/jimmunol.0903138. Epub 2010 Aug 2. J Immunol. 2010. PMID: 20679536
-
Ral: mediator of membrane trafficking.Int J Biochem Cell Biol. 2006;38(11):1841-7. doi: 10.1016/j.biocel.2006.04.006. Epub 2006 May 9. Int J Biochem Cell Biol. 2006. PMID: 16781882 Review.
-
Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function.Histochem Cell Biol. 2002 Feb;117(2):105-12. doi: 10.1007/s00418-001-0367-x. Epub 2002 Jan 22. Histochem Cell Biol. 2002. PMID: 11935286 Review.
Cited by
-
AtCAP2 is crucial for lytic vacuole biogenesis during germination by positively regulating vacuolar protein trafficking.Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1675-E1683. doi: 10.1073/pnas.1717204115. Epub 2018 Jan 29. Proc Natl Acad Sci U S A. 2018. PMID: 29378957 Free PMC article.
-
The Blood-prostate Barrier: An Obstacle to Drug Delivery into the Prostate.Curr Drug Deliv. 2025;22(4):401-412. doi: 10.2174/1567201821666230807152520. Curr Drug Deliv. 2025. PMID: 37550915 Review.
-
Caveolin-1 controls mitochondrial damage and ROS production by regulating fission - fusion dynamics and mitophagy.Redox Biol. 2022 Jun;52:102304. doi: 10.1016/j.redox.2022.102304. Epub 2022 Apr 6. Redox Biol. 2022. PMID: 35413643 Free PMC article.
-
The role of transcytosis in the blood-retina barrier: from pathophysiological functions to drug delivery.Front Pharmacol. 2025 Apr 16;16:1565382. doi: 10.3389/fphar.2025.1565382. eCollection 2025. Front Pharmacol. 2025. PMID: 40308764 Free PMC article. Review.
-
The RAL Enigma: Distinct Roles of RALA and RALB in Cancer.Cells. 2022 May 14;11(10):1645. doi: 10.3390/cells11101645. Cells. 2022. PMID: 35626682 Free PMC article. Review.
References
-
- Parton R. G., and del Pozo M. A. (2013) Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 14, 98–112 - PubMed
-
- Ahn S., Maudsley S., Luttrell L. M., Lefkowitz R. J., and Daaka Y. (1999) Src-mediated tyrosine phosphorylation of dynamin is required for β2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 274, 1185–1188 - PubMed
-
- Ahn S., Kim J., Lucaveche C. L., Reedy M. C., Luttrell L. M., Lefkowitz R. J., and Daaka Y. (2002) Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 277, 26642–26651 - PubMed
-
- Shajahan A. N., Timblin B. K., Sandoval R., Tiruppathi C., Malik A. B., and Minshall R. D. (2004) Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 279, 20392–20400 - PubMed
-
- Zimnicka A. M., Husain Y. S., Shajahan A. N., Sverdlov M., Chaga O., Chen Z., Toth P. T., Klomp J., Karginov A. V., Tiruppathi C., Malik A. B., and Minshall R. D. (2016) Src-dependent phosphorylation of caveolin-1 Tyr14 promotes swelling and release of caveolae. Mol. Biol. Cell 27, 2090–2106 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

