Pericytes for the treatment of orthopedic conditions
- PMID: 27510330
- PMCID: PMC6434715
- DOI: 10.1016/j.pharmthera.2016.08.003
Pericytes for the treatment of orthopedic conditions
Abstract
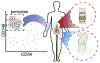
Pericytes and other perivascular stem cells are of growing interest in orthopedics and tissue engineering. Long regarded as simple regulators of angiogenesis and blood pressure, pericytes are now recognized to have MSC (mesenchymal stem cell) characteristics, including multipotentiality, self-renewal, immunoregulatory functions, and diverse roles in tissue repair. Pericytes are typified by characteristic cell surface marker expression (including αSMA, CD146, PDGFRβ, NG2, RGS5, among others). Although alone no marker is absolutely specific for pericytes, collectively these markers appear to selectively identify an MSC-like pericyte. The purification of pericytes is most well described as a CD146+CD34-CD45- cell population. Pericytes and other perivascular stem cell populations have been applied in diverse orthopedic applications, including both ectopic and orthotopic models. Application of purified cells has sped calvarial repair, induced spine fusion, and prevented fibrous non-union in rodent models. Pericytes induce these effects via both direct and indirect mechanisms. In terms of their paracrine effects, pericytes are known to produce and secrete high levels of a number of growth and differentiation factors both in vitro and after transplantation. The following review will cover existing studies to date regarding pericyte application for bone and cartilage engineering. In addition, further questions in the field will be pondered, including the phenotypic and functional overlap between pericytes and culture-derived MSC, and the concept of pericytes as efficient producers of differentiation factors to speed tissue repair.
Keywords: Bone; Cartilage; MSC; Mesenchymal stem cell; PSC; Perivascular stem cell.
Copyright © 2016. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest statement
K.T. and C.S. are inventors of perivascular stem cell-related patents filed from UCLA. K.T. and C.S. are founders of Scarless Laboratories, Inc. which sublicenses perivascular stem cell-related patents from the UC Regents, and who also hold equity in the company. C.S. is also an officer of Scarless Laboratories, Inc.
Figures






References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, & Begley DJ (2010). Structure and function of the blood-brain barrier. Neurobiol Dis. 37, 13–25. - PubMed
-
- Anderson HC, Hodges PT, Aguilera XM, Missana L, & Moylan PE (2000). Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J Histochem Cytochem 48, 1493–1502. - PubMed
-
- Anz AW, Hackel JG, Nilssen EC, & Andrews JR (2014). Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg 22, 68–79. - PubMed
-
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, … Betsholtz C (2010). Pericytes regulate the blood-brain barrier. Nature. 468, 557–561. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

