An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize
- PMID: 27510362
- PMCID: PMC5259581
- DOI: 10.1111/pbi.12611
An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize
Abstract
CRISPR/Cas9 is a powerful genome editing tool in many organisms, including a number of monocots and dicots. Although the design and application of CRISPR/Cas9 is simpler compared to other nuclease-based genome editing tools, optimization requires the consideration of the DNA delivery and tissue regeneration methods for a particular species to achieve accuracy and efficiency. Here, we describe a public sector system, ISU Maize CRISPR, utilizing Agrobacterium-delivered CRISPR/Cas9 for high-frequency targeted mutagenesis in maize. This system consists of an Escherichia coli cloning vector and an Agrobacterium binary vector. It can be used to clone up to four guide RNAs for single or multiplex gene targeting. We evaluated this system for its mutagenesis frequency and heritability using four maize genes in two duplicated pairs: Argonaute 18 (ZmAgo18a and ZmAgo18b) and dihydroflavonol 4-reductase or anthocyaninless genes (a1 and a4). T0 transgenic events carrying mono- or diallelic mutations of one locus and various combinations of allelic mutations of two loci occurred at rates over 70% mutants per transgenic events in both Hi-II and B104 genotypes. Through genetic segregation, null segregants carrying only the desired mutant alleles without the CRISPR transgene could be generated in T1 progeny. Inheritance of an active CRISPR/Cas9 transgene leads to additional target-specific mutations in subsequent generations. Duplex infection of immature embryos by mixing two individual Agrobacterium strains harbouring different Cas9/gRNA modules can be performed for improved cost efficiency. Together, the findings demonstrate that the ISU Maize CRISPR platform is an effective and robust tool to targeted mutagenesis in maize.
Keywords: Argonaute; anthocyaninless; CRISPR/Cas9; gene editing; maize; targeted mutagenesis.
© 2016 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
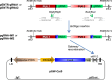

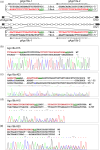

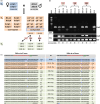
References
-
- Ausubel, F. , Brent, R. , Kingston, R. , Moore, D. , Seidman, J. and Struhl, K. (1993) Current Protocols in Molecular Biology, 1st ed. New York: Canada John Wiley and Sons.
-
- Bhaya, D. , Davison, M. and Barrangou, R. (2011) CRISPR‐Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 45, 273–297. - PubMed
-
- Callis, J. , Fromm, M. and Walbot, V. (1987) Introns increase gene expression in cultured maize cells. Genes Dev. 1, 1183–1200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

