Retinal Structures and Visual Cortex Activity are Impaired Prior to Clinical Vision Loss in Glaucoma
- PMID: 27510406
- PMCID: PMC4980591
- DOI: 10.1038/srep31464
Retinal Structures and Visual Cortex Activity are Impaired Prior to Clinical Vision Loss in Glaucoma
Abstract
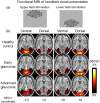
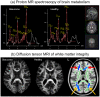
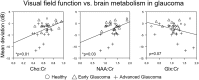
Glaucoma is the second leading cause of blindness worldwide and its pathogenesis remains unclear. In this study, we measured the structure, metabolism and function of the visual system by optical coherence tomography and multi-modal magnetic resonance imaging in healthy subjects and glaucoma patients with different degrees of vision loss. We found that inner retinal layer thinning, optic nerve cupping and reduced visual cortex activity occurred before patients showed visual field impairment. The primary visual cortex also exhibited more severe functional deficits than higher-order visual brain areas in glaucoma. Within the visual cortex, choline metabolism was perturbed along with increasing disease severity in the eye, optic radiation and visual field. In summary, this study showed evidence that glaucoma deterioration is already present in the eye and the brain before substantial vision loss can be detected clinically using current testing methods. In addition, cortical cholinergic abnormalities are involved during trans-neuronal degeneration and can be detected non-invasively in glaucoma. The current results can be of impact for identifying early glaucoma mechanisms, detecting and monitoring pathophysiological events and eye-brain-behavior relationships, and guiding vision preservation strategies in the visual system, which may help reduce the burden of this irreversible but preventable neurodegenerative disease.
Conflict of interest statement
The author(s) have made the following disclosure(s): J.S.S.: Royalties e Zeiss, Dublin, CA (for intellectual property licensed by the Massachusetts Institute of Technology and Massachusetts Eye and Ear Infirmary).
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

