Low μ-Opioid Receptor Status in Alcohol Dependence Identified by Combined Positron Emission Tomography and Post-Mortem Brain Analysis
- PMID: 27510425
- PMCID: PMC5240173
- DOI: 10.1038/npp.2016.145
Low μ-Opioid Receptor Status in Alcohol Dependence Identified by Combined Positron Emission Tomography and Post-Mortem Brain Analysis
Abstract
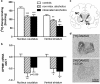
Blockade of the μ-opioid receptor (MOR) by naltrexone reduces relapse risk in a subpopulation of alcohol-dependent patients. Previous positron-emission-tomography (PET) studies using the MOR ligand [11C]carfentanil have found increased MOR availability in abstinent alcoholics, which may reflect either increased MOR expression or lower endogenous ligand concentration. To differentiate between both effects, we investigated two cohorts of alcoholic subjects using either post-mortem or clinical PET analysis. Post-mortem brain tissue of alcohol-dependent subjects and controls (N=43/group) was quantitatively analyzed for MOR ([3H]DAMGO)-binding sites and OPRM1 mRNA in striatal regions. [11C]carfentanil PET was performed in detoxified, medication free alcohol-dependent patients (N=38), followed by a randomized controlled study of naltrexone versus placebo and follow-up for 1 year (clinical trial number: NCT00317031). Because the functional OPRM1 variant rs1799971:A>G affects the ligand binding, allele carrier status was considered in the analyses. MOR-binding sites were reduced by 23-51% in post-mortem striatal tissue of alcoholics. In the PET study, a significant interaction of OPRM1 genotype, binding potential (BPND) for [11C]carfentanil in the ventral striatum, and relapse risk was found. Particularly in G-allele carriers, lower striatal BPND was associated with a higher relapse risk. Interestingly, this effect was more pronounced in the naltrexone treatment group. Reduced MOR is interpreted as a neuroadaptation to an alcohol-induced release of endogenous ligands in patients with severe alcoholism. Low MOR availability may explain the ineffectiveness of naltrexone treatment in this subpopulation. Finally, low MOR-binding sites are proposed as a molecular marker for a negative disease course.
Figures

Similar articles
-
Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil.Arch Gen Psychiatry. 2005 Jan;62(1):57-64. doi: 10.1001/archpsyc.62.1.57. Arch Gen Psychiatry. 2005. PMID: 15630073
-
Association of smoking with μ-opioid receptor availability before and during naltrexone blockade in alcohol-dependent subjects.Addict Biol. 2014 Jul;19(4):733-42. doi: 10.1111/adb.12022. Epub 2012 Dec 18. Addict Biol. 2014. PMID: 23252742 Free PMC article.
-
Nicotine-specific and non-specific effects of cigarette smoking on endogenous opioid mechanisms.Prog Neuropsychopharmacol Biol Psychiatry. 2016 Aug 1;69:69-77. doi: 10.1016/j.pnpbp.2016.04.006. Epub 2016 Apr 17. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 27095017 Free PMC article.
-
Systematic review and meta-analysis of the moderating effect of rs1799971 in OPRM1, the mu-opioid receptor gene, on response to naltrexone treatment of alcohol use disorder.Addiction. 2020 Aug;115(8):1426-1437. doi: 10.1111/add.14975. Epub 2020 Feb 11. Addiction. 2020. PMID: 31961981 Free PMC article.
-
Ligand-Free Signaling of G-Protein-Coupled Receptors: Relevance to μ Opioid Receptors in Analgesia and Addiction.Molecules. 2022 Sep 8;27(18):5826. doi: 10.3390/molecules27185826. Molecules. 2022. PMID: 36144565 Free PMC article. Review.
Cited by
-
Neuroimaging of reward mechanisms in Gambling disorder: an integrative review.Mol Psychiatry. 2019 May;24(5):674-693. doi: 10.1038/s41380-018-0230-2. Epub 2018 Sep 13. Mol Psychiatry. 2019. PMID: 30214041 Review.
-
Dysregulation of the histone demethylase KDM6B in alcohol dependence is associated with epigenetic regulation of inflammatory signaling pathways.Addict Biol. 2021 Jan;26(1):e12816. doi: 10.1111/adb.12816. Epub 2019 Aug 1. Addict Biol. 2021. PMID: 31373129 Free PMC article.
-
Endogenous mu-opioid modulation of social connection in humans: a systematic review and meta-analysis.Transl Psychiatry. 2024 Sep 17;14(1):379. doi: 10.1038/s41398-024-03088-3. Transl Psychiatry. 2024. PMID: 39289345 Free PMC article.
-
Risk for opioid misuse in chronic pain patients is associated with endogenous opioid system dysregulation.Transl Psychiatry. 2022 Jan 12;12(1):20. doi: 10.1038/s41398-021-01775-z. Transl Psychiatry. 2022. PMID: 35022382 Free PMC article.
-
Dynorphin and κ-Opioid Receptor Dysregulation in the Dopaminergic Reward System of Human Alcoholics.Mol Neurobiol. 2018 Aug;55(8):7049-7061. doi: 10.1007/s12035-017-0844-4. Epub 2018 Jan 30. Mol Neurobiol. 2018. PMID: 29383684 Free PMC article.
References
-
- Anton RF, Oroszi G, O'malley S, Couper D, Swift R, Pettinati H et al (2008). An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry 65: 135–144. - PMC - PubMed
-
- Bach P, Vollsta Dt-Klein S, Kirsch M, Hoffmann S, Jorde A, Frank J et al (2015). Increased mesolimbic cue-reactivity in carriers of the mu-opioid-receptor gene OPRM1 A118G polymorphism predicts drinking outcome: a functional imaging study in alcohol dependent subjects. Eur Neuropsychopharmacol 25: 1128–1135. - PubMed
-
- Bilbao A, Robinson JE, Heilig M, Malanga CJ, Spanagel R, Sommer WH, Thorsell A (2015). A pharmacogenetic determinant of mu-opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice. Biol Psychiatry 77: 850–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

