Predictors of failure of fish-oil therapy for intestinal failure-associated liver disease in children
- PMID: 27510535
- PMCID: PMC4997303
- DOI: 10.3945/ajcn.116.137083
Predictors of failure of fish-oil therapy for intestinal failure-associated liver disease in children
Abstract
Background: Parenteral fish-oil (FO) therapy is a safe and effective treatment for intestinal failure-associated liver disease (IFALD). Patients whose cholestasis does not resolve with FO may progress to end-stage liver disease.
Objective: We sought to identify factors associated with the failure of FO therapy in treating IFALD to guide prognostication and referral guidelines.
Design: Prospectively collected data for patients treated with FO at Boston Children's Hospital from 2004 to 2014 were retrospectively reviewed. Resolution of cholestasis was defined as sustained direct bilirubin (DB) <2 mg/dL, and treatment failure as liver transplantation or death while DB was >2 mg/dL as of July 2015. Demographics, laboratory values, and medical history at FO therapy initiation were compared between patients who achieved resolution of cholestasis and those who failed therapy.
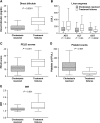
Results: Among 182 patients treated with FO, 86% achieved resolution of cholestasis and 14% failed therapy. Patients who failed therapy had median (IQR) lower birth weight [1020 g (737, 1776 g) compared with 1608 g (815, 2438 g); P = 0.03] and were older at FO initiation [20.4 wk (9.9, 38.6 wk) compared with 11.7 wk (7.3, 21.4 wk); P = 0.02] than patients whose cholestasis resolved. Patients who failed therapy had more advanced liver disease at therapy initiation than patients whose cholestasis resolved, as evidenced by lower median (IQR) γ-glutamyltransferase [54 U/L (41, 103 U/L) compared with 112 U/L (76, 168 U/L); P < 0.001], higher DB [10.4 mg/dL (7.5, 14.1 mg/dL) compared with 4.4 mg/dL (3.1, 6.6 mg/dL); P < 0.001], and a higher pediatric end-stage liver disease (PELD) score [22 (14, 25) compared with 12 (7, 15); P < 0.001]. A PELD score of ≥15, history of gastrointestinal bleeding, age at FO initiation ≥16 wk, presence of nongastrointestinal comorbidities, and mechanical ventilation at FO initiation were independent predictors of treatment failure.
Conclusions: Most infants with IFALD responded to FO therapy with resolution of cholestasis, and liver transplantation was rarely required. Early FO initiation once biochemical cholestasis is detected in parenteral nutrition-dependent patients is recommended. This trial was registered at clinicaltrials.gov as NCT00910104.
Keywords: Omegaven; cholestasis; fish oil; fish oil lipid emulsion; intestinal failure; intestinal failure–associated liver disease; parenteral fish oil; parenteral nutrition; parenteral nutrition–associated liver disease.
© 2016 American Society for Nutrition.
Figures


References
-
- Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery 1968;64:134–42. - PubMed
-
- Wilmore DW, Dudrick SJ. Growth and development of an infant receiving all nutrients exclusively by vein. JAMA 1968;203:860–4. - PubMed
-
- Fallon EM, Mitchell PD, Nehra D, Potemkin AK, O’Loughlin AA, Gura KM, Puder M. Neonates with short bowel syndrome: an optimistic future for parenteral nutrition independence. JAMA Surg 2014;149:663–70. - PubMed
-
- Mullick FG, Moran CA, Ishak KG. Total parenteral nutrition: a histopathologic analysis of the liver changes in 20 children. Mod Pathol 1994;7:190–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

