Genomic variation within alpha satellite DNA influences centromere location on human chromosomes with metastable epialleles
- PMID: 27510565
- PMCID: PMC5052062
- DOI: 10.1101/gr.206706.116
Genomic variation within alpha satellite DNA influences centromere location on human chromosomes with metastable epialleles
Abstract
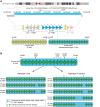
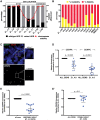
Alpha satellite is a tandemly organized type of repetitive DNA that comprises 5% of the genome and is found at all human centromeres. A defined number of 171-bp monomers are organized into chromosome-specific higher-order repeats (HORs) that are reiterated thousands of times. At least half of all human chromosomes have two or more distinct HOR alpha satellite arrays within their centromere regions. We previously showed that the two alpha satellite arrays of Homo sapiens Chromosome 17 (HSA17), D17Z1 and D17Z1-B, behave as centromeric epialleles, that is, the centromere, defined by chromatin containing the centromeric histone variant CENPA and recruitment of other centromere proteins, can form at either D17Z1 or D17Z1-B. Some individuals in the human population are functional heterozygotes in that D17Z1 is the active centromere on one homolog and D17Z1-B is active on the other. In this study, we aimed to understand the molecular basis for how centromere location is determined on HSA17. Specifically, we focused on D17Z1 genomic variation as a driver of epiallele formation. We found that D17Z1 arrays that are predominantly composed of HOR size and sequence variants were functionally less competent. They either recruited decreased amounts of the centromere-specific histone variant CENPA and the HSA17 was mitotically unstable, or alternatively, the centromere was assembled at D17Z1-B and the HSA17 was stable. Our study demonstrates that genomic variation within highly repetitive, noncoding DNA of human centromere regions has a pronounced impact on genome stability and basic chromosomal function.
© 2016 Aldrup-MacDonald et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
